EXHIBIT 99.1
Published on April 19, 2018

2017
Annual
Report
One Company
One Culture
One Mission

Becoming the World’s Leading Cancer
Testing and Information Company
OUR COMMON PURPOSE
We save lives by improving patient care.
OUR VISION
By providing uncompromising quality, exceptional
service and innovative solutions, we will be the world’s
leading cancer testing and information company.
OUR VALUES
Quality, Integrity, Accountability,
Teamwork, and Innovation
• Grew Clinical Test volume 17% year-over-year,
performing over 650,000 complex clinical oncology
tests for approximately 400,000 cancer patients.
• Grew Pharma Services revenue 22% year-over-year
and increased backlog from $36 million to over
$66 million.
• Improved cost effectiveness with 11% cost-per-
test reduction fueled by a productivity increase of
over 7% and continued automation and process
improvements.
2017 Results
2017 Payer Mix
• Expanded outside the U.S. by opening a new
laboratory in Rolle, Switzerland offering Pharma
Services to international clients.
• Completed the integration of Clarient, combining
our California laboratories and renovating our
large laboratory in Aliso Viejo, California, to
enhance testing capacity.
Key 2017 Accomplishments
2017 Revenue by Segment
Pharma
Services
10%
Clinical Services
90%
2017 Test Mix
Cyto
8%
FISH
23%
Flow
13%
Molecular
23%
Histology &
Pathology
33%
Patient &
Other
3%
Client Billing
64%
Govt.
15%
Insurance
18%
NeoGenomics Laboratories • 1
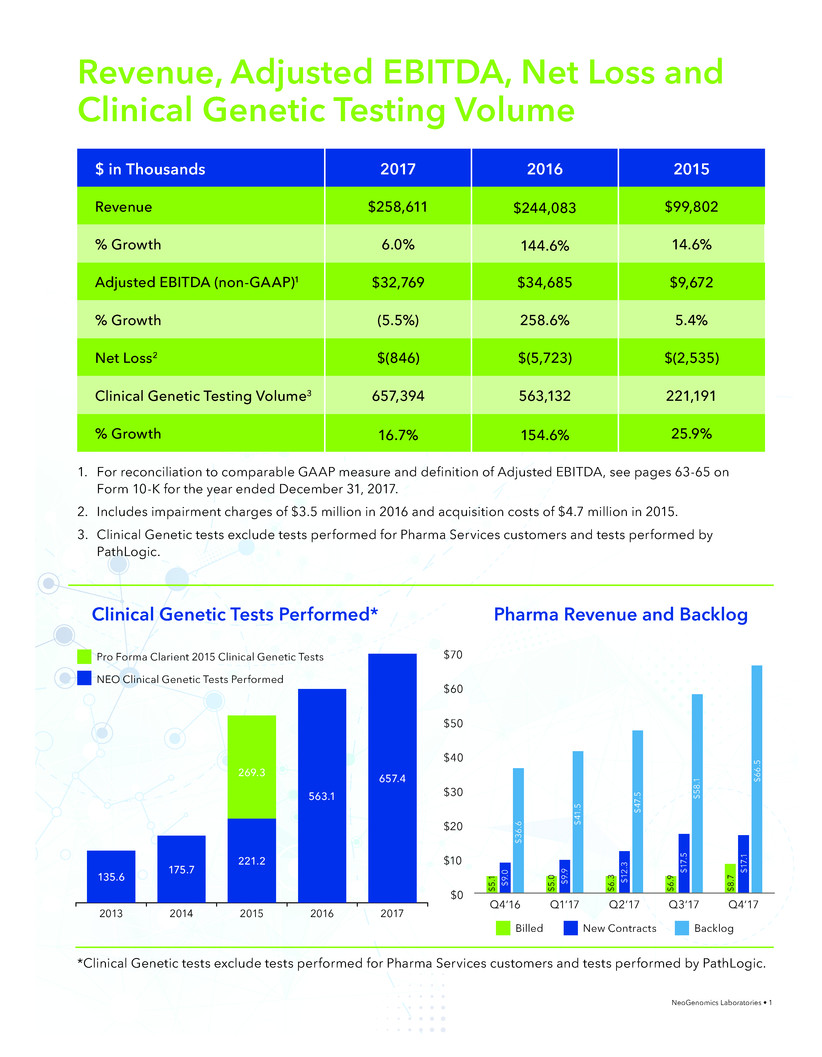
Revenue, Adjusted EBITDA, Net Loss and
Clinical Genetic Testing Volume
Clinical Genetic Tests Performed* Pharma Revenue and Backlog
$ in Thousands 2017 2016 2015
Revenue $258,611 $244,083 $99,802
% Growth 6.0% 144.6% 14.6%
Adjusted EBITDA (non-GAAP)1 $32,769 $34,685 $9,672
% Growth (5.5%) 258.6% 5.4%
Net Loss2 $(846) $(5,723) $(2,535)
Clinical Genetic Testing Volume3 657,394 563,132 221,191
% Growth 16.7% 154.6% 25.9%
1. For reconciliation to comparable GAAP measure and definition of Adjusted EBITDA, see pages 63-65 on
Form 10-K for the year ended December 31, 2017.
2. Includes impairment charges of $3.5 million in 2016 and acquisition costs of $4.7 million in 2015.
3. Clinical Genetic tests exclude tests performed for Pharma Services customers and tests performed by
PathLogic.
*Clinical Genetic tests exclude tests performed for Pharma Services customers and tests performed by PathLogic.
Billed New Contracts Backlog
2013 2014 2015 2016 2017
135.6
175.7
221.2
269.3
563.1
657.4
Pro Forma Clarient 2015 Clinical Genetic Tests
NEO Clinical Genetic Tests Performed
$70
$60
$50
$40
$30
$20
$10
$0
Q4’16 Q1’17 Q2’17 Q3’17 Q4’17
$
5
.1
$
5
.0
$
6
.3
$
6
.9
$
8
.7$9
.0
$9
.9
$1
2
.3 $1
7.
5
$1
7.
1
$
3
6
.6 $
41
.5 $
47
.5 $
5
8
.1 $
6
6
.5

Dear Fellow Shareholders:
We live in an exciting time – advances in science and medicine are rapidly changing our
understanding of Oncology, and are creating treatments and hope for patients. NeoGenomics
provides the essential, state-of-the-art, genomic testing which allows physicians to treat
patients more precisely and effectively than ever before. This is our purpose and why we exist.
Our company has evolved rapidly into one of the leaders in Oncology testing. We have the
most comprehensive menu for somatic cancer testing in our industry, a workforce which is
unparrelled in its Oncology testing skill and expertise, low-cost operations driven by our scale
and process management, and a loyal customer base supported by world-class service. We are
positioned well and are stronger than ever.
2017 was a year of fortification. We significantly strengthened our company by completing the
integration of Clarient while retaining nearly 100% of our clients. At the same time, we grew
organically and gained market share in our Clinical and Pharma Services Divisions.
We exited 2017 as an International company with exciting growth prospects. Our momentum
is strong and we are committed to be the World’s leading cancer testing and information
company.
Culture and Values
We are a purpose-driven company with a core set of Values. We know that patient lives depend
on us and our clients place great trust in us. We know that Quality, Integrity, Accountability,
Teamwork and Innovation make us strong. And we know that our success requires us to
provide uncompromising quality, exceptional service and innovative solutions. These ideals
are foundational elements of our company and they drive our success.
Rapid Growth
NeoGenomics operates two Oncology testing Divisions: Our Clinical Services Division, and
our Pharma Services Division. Each grew significantly in 2017.
Clinical Services test volumes increased 17% and revenues grew 6% year-over-year as we
gained market share. Our comprehensive test menu, which provides a “one-stop shop” for
hospitals and Oncology groups, together with our strong service levels, have generated more
demand for our services. Growth accelerated in the second half of the year and demand from
larger clients increased. This momentum is very encouraging as we enter 2018.
Revenue growth in Pharma Services accelerated as the year progressed. Revenue increased
22% year-over-year, with fourth quarter growth exceeding 60%. The significant increase
in backlog of signed contracts, growing from $36 million to $66 million, demonstrates our
momentum. Pharma Services is extremely important as it allows us to partner with the most
innovative companies in the industry and assist in their drug development programs. It also
provides us with key insights into leading developments in Oncology.
Investments in laboratory facilities, instrumentation, and automation have ensured capacity for
future growth. We expanded our California laboratory facilities and opened a new laboratory
in Rolle, Switzerland this past year. We also began investment in a new Houston laboratory,
which is scheduled to open in May 2018.
Growth in our business is also driven by innovation and we continue to lead in this area. Our
expanding menu of multi-modality tests combine the most state-of-the-art testing techniques
for use by Physicians and researchers. In the rapidly-growing field of immuno-oncology, we
provide a large percentage of America’s PD-L1 testing along with a rapidly-growing molecular
testing capability and proprietary testing techniques.
While the incidence of cancer continues to increase, new diagnostic and therapeutic capabilities
are increasing at an even faster rate. NeoGenomics is committed to lead and provide growth
opportunities for many years to come.
NeoGenomics Laboratories • 2

World-Class Team
We are fortunate to have a talented and dedicated team of employees that drive our Company’s
achievements. Together, we form a world-class team of over 1,000 employees and contracted
Pathologists, including a medical and scientific team with diverse backgrounds in specialties
related to genetics, pathology and oncology.
Continual development of our team is important to us. We also mentor and train our people to
enhance and capitalize on the talent within our Company. We believe that a culture of motivated
and engaged employees will deliver superior service to our customers.
Service Excellence
Cancer patients and their Physicians demand service excellence and we agree that they
should. We pride ourselves on the consistency of our service. Our industry leading turnaround
times and consistent timeliness of results differentiates us from other laboratories and allows
patients to receive treatment sooner. Our service excellence is demonstrated by our high levels
of customer retention, which stood at 97.5% over the past year. Adjusting for clients that were
acquired and similar circumstances, that retention rate would be closer to 99%. Customer
survey results also provide evidence that NeoGenomics’ services are delivering very high levels
of customer satisfaction. We know there is a cancer patient behind every sample we receive
and our team takes that to heart at every step in the process.
Challenges
All businesses experience challenges and NeoGenomics is no exception. Reimbursement is
the most significant challenge we face. We work hard to provide testing that is value added and
medically necessary, but in many cases it’s difficult to get reimbursed adequately, or at all, for
the work we do. We are working closely with payers, regulators, and legislators to drive more
sensible reimbursement practices.
We believe that the combined efforts of providers, payers, researchers, patients, and legislators
will enable wonderful advances in Oncology care to continue, and we are committed to do our part.
Future
We are excited about the future of NeoGenomics and the many opportunities we have to grow
and create value for our society. We expect the strong momentum we built in 2017 to continue
into 2018. The Oncology testing market is growing and exciting new discoveries, developments
and treatments are being introduced almost daily. Our leadership team is dedicated to
pursuing our mission and to provide the exceptional quality, service and innovation necessary
to increase our market share and grow profitably.
This is a great time to be a part of NeoGenomics, and we are committed to lead. On behalf of
everyone at NeoGenomics, thank you for your continued support and confidence.
Sincerely,
Douglas M. VanOort
Chairman of the Board of
Directors and Chief Executive Officer
NeoGenomics Laboratories • 3
NeoGenomics Laboratories • 4
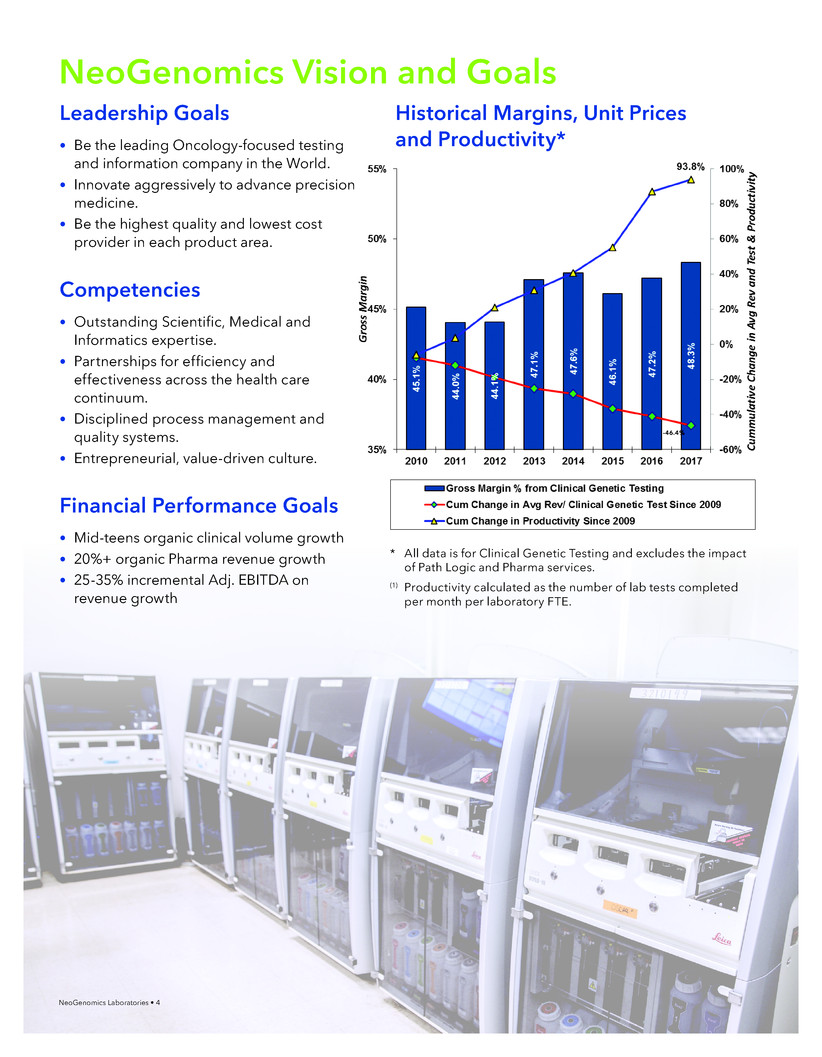
NeoGenomics Vision and Goals
Leadership Goals
• Be the leading Oncology-focused testing
and information company in the World.
• Innovate aggressively to advance precision
medicine.
• Be the highest quality and lowest cost
provider in each product area.
Competencies
• Outstanding Scientific, Medical and
Informatics expertise.
• Partnerships for efficiency and
effectiveness across the health care
continuum.
• Disciplined process management and
quality systems.
• Entrepreneurial, value-driven culture.
Financial Performance Goals
• Mid-teens organic clinical volume growth
• 20%+ organic Pharma revenue growth
• 25-35% incremental Adj. EBITDA on
revenue growth
* All data is for Clinical Genetic Testing and excludes the impact
of Path Logic and Pharma services.
(1) Productivity calculated as the number of lab tests completed
per month per laboratory FTE.
Historical Margins, Unit Prices
and Productivity*

Corporate Information
Board of Directors
• Bruce K. Crowther
• Director
• Kevin C. Johnson
• Director
• Douglas M. VanOort
• Chairman and CEO
• Alison L. Hannah
• Director
• Steven C. Jones
• Director
• William J. Robison
• Director
• Raymond R. Hipp
• Director
• Stephen M. Kanovsky
• Director
• Lynn A. Tetrault
• Director
Corporate Officers
Jennifer M. Balliet
Chief Culture Officer
Kathryn B. McKenzie
Principal Accounting
Officer
William B. Bonello
VP of Corporate Development,
Strategy & Investor Relations
Steven A. Ross
Chief Information Officer
Steven G. Brodie, PhD
Vice President of Operations
Robert J. Shovlin
President, Clinical Services
George A. Cardoza
President, Pharma Services
Sharon A. Virag
Chief Financial Officer
Corporate Offices
12701 Commonwealth Drive, Suite 9
Fort Myers, FL 33913
Legal Counsel
K&L Gates, LLP, Miami, FL
Transfer Agent
Standard Registrar and Transfer Company
440 East 400 South, Suite 200
Salt Lake City, UT 84111
Phone: 801.596.2150
Independent Registered Public Accounting Firm
Crowe Horwath LLP
Indianapolis, Indiana
Annual Meeting
The annual meeting of stockholders will be held at the
Hyatt Coconut Point Resort at: 5001 Coconut Road Bonita
Springs, FL 34134 on June 1, 2018 at 10:00am EDT.
Stock Listing and Information
The Company’s stock trades under the symbol “NEO” on the
NASDAQ Capital Market.
Forward Looking Statements
Certain information contained in this annual report
constitutes forward-looking statements for purposes of the
safe harbor provisions of The Private Securities Litigation
Reform Act of 1995, including the information set forth in the
Letter to Shareholders. These forward-looking statements
involve a number of risks and uncertainties that could cause
actual future results to differ materially from those anticipated
in the forward-looking statements as the result of the
Company’s ability to continue gaining new customers, offer
new types of tests, integrate its acquisition of the Clarient
business, and otherwise implement its business plan, as
well as additional factors discussed under the heading “Risk
Factors” contained in Item 1A in our Annual Report on Form
10-K for the year ended December 31, 2017, filed with the
Securities and Exchange Commission on March 13, 2018. All
information in the Letter to Shareholders is as of release and
should not be relied upon as representing the Company’s
estimates as of any subsequent date. While the Company
may elect to update forward-looking statements at some
point in the future, it specifically disclaims any obligation to
do so, even if its estimates change.
Medical Leadership
Maher Albitar, MD
Chief Medical Officer & Director of R & D
Sally Agersborg, MD & PhD
Director of Hematopathology
Josette William Ragheb, MD & PhD
Medical Director
Adrian Padurean, MD
Medical Director
Lawrence M. Weiss, MD
Medical Director

NeoGenomics Laboratories is a specialized oncology reference laboratory providing the latest technologies, testing
partnership opportunities, and interactive education to the oncology and pathology communities. We offer the complete
spectrum of diagnostic services in molecular testing, FISH, cytogenetics, flow cytometry, and immunohistochemistry
through our nation-wide network of CAP-accredited, CLIA-certified laboratories.
Committed to research as the means to improve patient care, we provide Pharma Services for pharmaceutical
companies, in vitro diagnostic manufacturers, and academic scientist-clinicians. We promote joint publications with our
client physicians. NeoGenomics welcomes your inquiries for collaborations. Please contact us for more information.
12701 Commonwealth Dr., Suite 9
Fort Myers, FL 33913
Phone: 866.776.5907/ Fax: 239.690.4237
neogenomics.com
© 2018 NeoGenomics Laboratories, Inc. All Rights Reserved.
All other trademarks are the property of their respective owners.
Rev. 041318