EXHIBIT 99.1
Published on April 26, 2019

Exhibit 99.1 2018 Annual Report

Becoming the World’s Leading Cancer Testing and Information Company OUR COMMON PURPOSE We save lives by improving patient care. OUR VISION By providing uncompromising quality, exceptional service and innovative solutions, we will be the world’s leading cancer testing and information company. OUR VALUES Quality, Integrity, Accountability, Teamwork, and Innovation Key 2018 Strategic Accomplishments Key 2018 Financial Highlights • Acquired Genoptix, adding critical scale • Performed approximately 750,000 clinical and complimentary capabilities to serve oncology tests for approximately 440,000 community oncology practices. cancer patients. • Entered a Global Strategic Alliance • Grew consolidated revenues 15.2% year- with PPD, a leading Contract Research over-year to $276.7 million, with clinical test Organization (CRO) to expand global volumes up 14.1% and average revenue per Pharma services reach. clinical test up 1.2%. • Entered into a national agreement • Grew Pharma services revenue 28.4% year- with Cigna Corporation to become a over-year to $34.9 million and increased participating in-network provider for all backlog by 44.0% to $98.9 million. Cigna health insurance products. • Improved cost efficiency and productivity, • Entered a strategic alliance with Qiagen achieving a 4.5% reduction in cost per test to offer Day-One access to innovative and a 30% increase in adjusted EBITDA to companion diagnostics for newly $43.6 million. approved drugs. • Successfully completed a $135 million, net public offering of common stock. • Redeemed 100% of Series A Redeemable Preferred stock.
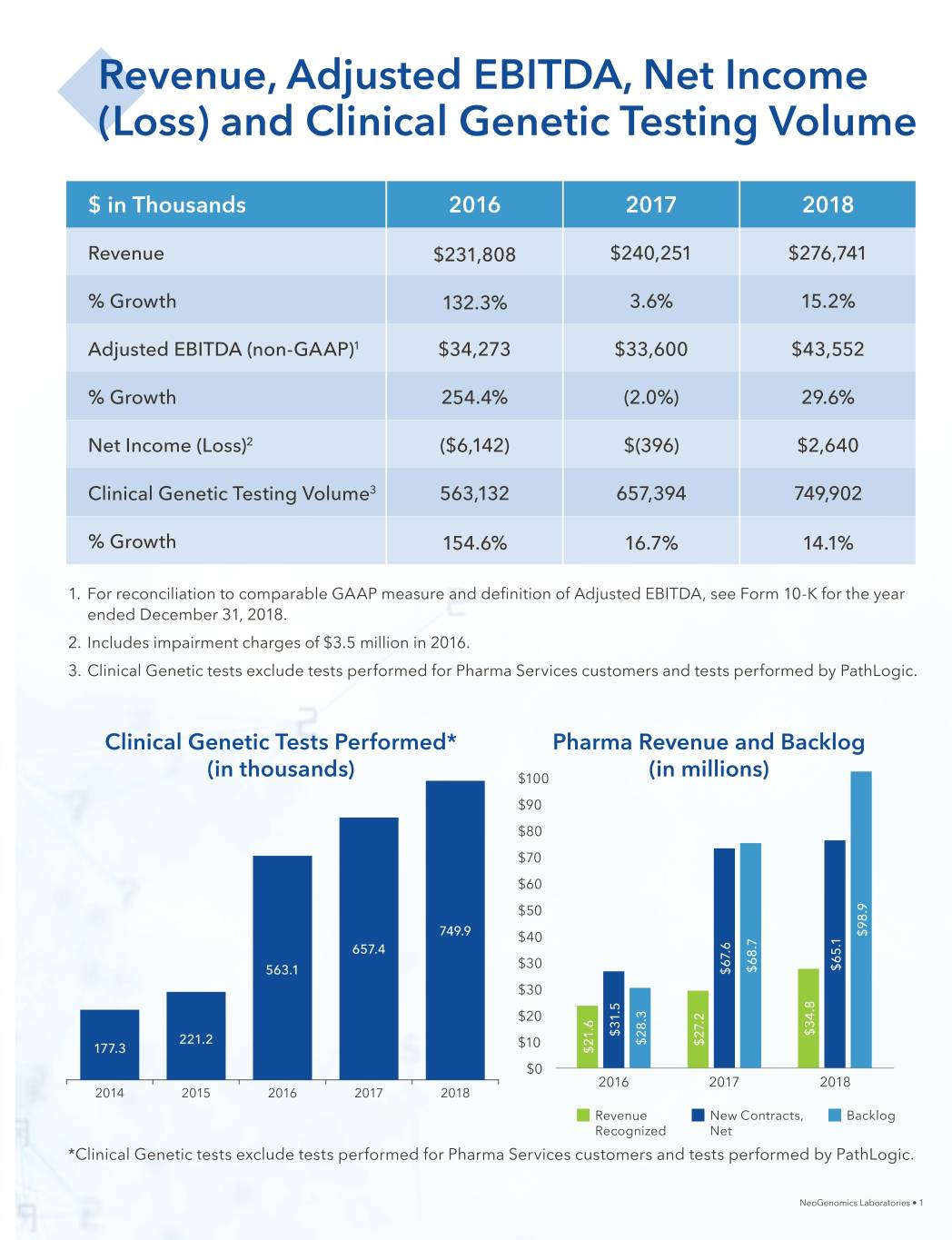
Revenue, Adjusted EBITDA, Net Income (Loss) and Clinical Genetic Testing Volume $ in Thousands 2016 2017 2018 Revenue $231,808 $240,251 $276,741 % Growth 132.3% 3.6% 15.2% Adjusted EBITDA (non-GAAP)1 $34,273 $33,600 $43,552 % Growth 254.4% (2.0%) 29.6% Net Income (Loss)2 ($6,142) $(396) $2,640 Clinical Genetic Testing Volume3 563,132 657,394 749,902 % Growth 154.6% 16.7% 14.1% 1. For reconciliation to comparable GAAP measure and definition of Adjusted EBITDA, see Form 10-K for the year ended December 31, 2018. 2. Includes impairment charges of $3.5 million in 2016. 3. Clinical Genetic tests exclude tests performed for Pharma Services customers and tests performed by PathLogic. Clinical Genetic Tests Performed* Pharma Revenue and Backlog (in thousands) $100 (in millions) $90 $80 $70 $60 $50 749.9 $40 $98.9 657.4 $65.1 $30 $68.7 563.1 $67.6 $30 $20 $34.8 $31.5 $28.3 221.2 $10 $27.2 177.3 $21.6 $0 2016 2017 2018 2014 2015 2016 2017 2018 Revenue New Contracts, Backlog Recognized Net *Clinical Genetic tests exclude tests performed for Pharma Services customers and tests performed by PathLogic. NeoGenomics Laboratories • 1

Dear Fellow Shareholders: Earlier this year, at a large annual management meeting with about 150 of our employees and members of our Board of Directors, we listened to a guest speaker named Rachael. A former Olympic athlete, young mother of one child, and a working professional, Rachael is also a survivor of non-small-cell lung cancer. As she explained her journey, she told us that testing performed by NeoGenomics is the reason she is alive and that our test results helped her doctors identify treatment pathways they hadn’t previously considered. Although I did not see many dry eyes, I did see a deep-set commitment on the faces of the NeoGenomics people in that room. Rachael’s story helps explain our common purpose at NeoGenomics. It explains why our people are so passionate about our work. It explains why we are excited to be at the forefront of advances in diagnostic medicine and technology. It explains why we constantly seek new ways to improve our performance. It explains why we value Quality, Accountability, Integrity, Teamwork, and Innovation. It explains our Culture. As you read this letter describing our accomplishments, strong growth and performance in 2018, please recognize the employees of NeoGenomics. They are the reason why our company is becoming a global leader in Oncology diagnostics, and why our future is so bright. Competitive Positioning 2018 was a year in which we began to “Bend the Curve”, positioning the company for accelerating growth and enhanced profitability in the years to come. The list of accomplishments is long. We entered into global strategic alliances with PPD and QIAGEN, signed national managed care and GPO contracts with Cigna and Premier, were selected by the National Cancer Institute to participate in NCI-Match, and significantly enhanced our quality, regulatory, reimbursement and informatics capabilities. And importantly, we acquired Genoptix, a leading oncology laboratory with an outstanding reputation among community oncology practices. We ended 2018 with strong momentum, competitively and uniquely positioned to serve oncologists, pathologists, hospitals and biopharmaceutical companies as a leader in cancer diagnostics. Genoptix On December 10th, we completed the acquisition of Genoptix. This was an important strategic acquisition for our company. Our combination with Genoptix sets NeoGenomics apart from the rest of the industry with an unprecedented reach to all clinical customer segments, including hospitals, pathologists, and community oncology practices. The combination also increases our competitiveness by leveraging the best offerings and capabilities from each company. Together, we have a large and highly specialized Medical Team of approximately 80 oncology-specialized pathologists, hematopathologists, and PhDs, the industry’s broadest and deepest oncology-focused test menu, and specialized pathology consults and customized oncology test reports. In addition, we have a broad portfolio of managed care and Hospital Group Purchasing Organization contracts and an 80+ person oncology-focused sales force. Financially, this combination bends the curve by significantly accelerating our revenue and profit trajectory. We expect Genoptix to add approximately $80 to $85 in million revenue in 2019, and drive increased profitability over time. In addition to the long-term revenue growth opportunities, we have identified $25 million of annual cost synergies which we expect to fully realize by the end of 2021. Strategic Collaborations In 2018 we announced several important strategic collaborations to better serve our customers and advance our position in the market, including: • A Global Strategic Alliance with PPD, a leading global contract research organization (CRO) providing a seamless and fully integrated global pathology and molecular testing solution to PPD’s pharmaceutical and biotech clients. NeoGenomics Laboratories • 2

• A national agreement with Cigna Corporation becoming a participating in-network provider for all Cigna health insurance products. • A group purchasing agreement for clinical reference laboratory testing services with Premier, a leading healthcare improvement company, uniting an alliance of approximately 4,000 U.S. hospitals and 165,000 other providers to transform healthcare. • Participation as a designated laboratory in the NCI-Molecular Analysis for Therapy Choice (NCI-MATCH or EAY131) precision medicine cancer treatment clinical trial. NeoGenomics was selected based on peer review of the validation, reliability and accuracy of our NeoTYPE Discovery Panel. • An agreement with QIAGEN to accelerate the availability of innovative companion diagnostics that enable precision medicine for cancer patients. People Maintaining world class culture is critical to our success and remains a core commitment. In 2018, we focused on developing our employees and advancing their careers. Our employee retention rates continued to improve and reached all-time highs in 2018. In addition to capitalizing on the talent within our company, we worked hard to improve our ability to attract employees as we hired over 300 people into our company to support growth. In particular, we added to our quality and regulatory teams, strengthened our marketing team, grew our medical team, and expanded our sales force. The acquisition of Genoptix brought a group of highly-skilled people to NeoGenomics’ workforce, which now totals approximately 1,500 employees globally. We are particularly excited about the depth of knowledge and capability of the Genoptix molecular and Medical teams. As we progressed through the integration process, we gained an understanding of the opportunity to realize synergies drawing upon the best people and practices of each organization to form a new and more powerful capability going forward. We also announced several important additions strengthening our executive management team in 2018. • Sharon A. Virag as Chief Financial Officer. Sharon was formerly Vice President, Corporate Finance and Chief Accounting Officer at Aetna, with responsibility for Controllership, Tax, Treasury, Finance Transformation and Finance Shared Services. Sharon’s outstanding financial leadership skills and experience, coupled with her strong alignment with our NeoGenomics culture, have already proven invaluable to us as we continue to grow and further develop our company. • George A. Cardoza as President of the Pharma Services Division. George had served as our CFO since 2009 and brings an excellent understanding of operational and strategic imperatives as the leader of our fast-growing Pharma Services Division. George has already made a significant impact on this business, evidenced by the strong revenue growth, healthy backlog of signed contracts, and emphasis on quality and culture achieved in 2018. • Lawrence M. Weiss, M.D. as Chief Scientific Officer. Dr. Weiss and has served as Medical Director and Laboratory Director for NeoGenomics Laboratories since joining NeoGenomics as part of the Clarient acquisition in 2015. Dr. Weiss is a highly-regarded thought-leader in the oncology and pathology community, with years of medical and scientific leadership experience at NeoGenomics and other organizations. As Chief Scientific Officer, Dr. Weiss is responsible for driving all-important scientific innovation initiatives and advancing our reputation in the oncology diagnostics community. NeoGenomics Laboratories • 3

Exceptional Service and Growth 2018 was a year of significant growth. We increased revenue 15.2% to $276.7 million driven by organic growth and a modest contribution from the acquisition of Genoptix late in the year. Clinical Services test volumes increased 14% compared to 2017 and revenue per test improved slightly at 1%. Pharma Services revenue increased 28% and the Pharma Services backlog increased 44% year over year to nearly $100 million. Our commitment to grow profitably continued in 2018. Cost per test declined 4.6% year-over-year, driving a 360 basis point gross margin improvement. Additionally, we posted 2018 Adjusted EBITDA of $43.6M which represents 30% growth year over year, and our Adjusted EBITDA Margin expanded 170 basis points versus 2017 to nearly 16%. We achieved this growth while maintaining outstanding levels of customer service. Client satisfaction for both divisions, measured by Net Promoter Scores, were considered world class, and client retention rates continued to be above 98%. We also continued to expand our global footprint, relocating and expanding our Houston lab as a flagship facility for our Pharma Services Division, opening a new Flow Cytometry-focused laboratory in Atlanta Georgia, building out a new Pharma Services lab in Singapore and adding two additional labs in Southern California as part of the Genoptix acquisition. Strengthened Financial Position We also made substantial progress to enhance our financial position in 2018. • In June, we redeemed 6,864,000 shares of Series A Redeemable Preferred Stock (“Series A Preferred Stock”) held by a subsidiary of General Electric Company for approximately $50.1 million, reducing our Adjusted Diluted Shares outstanding by approximately 8%. We financed this redemption by securing a $30 million extension to our senior secured credit facility and with internally generated operating cash flow. In less than 30 months since completing the Clarient acquisition, we redeemed 100% of the $110 million preferred stock issued in conjunction with the deal, at a total redemption cost of just $105 million. • In August, we issued 11,270,000 shares of common stock at a public offering price of $12.75 per share. Net proceeds to NeoGenomics from the offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by NeoGenomics, were $135.1 million. Proceeds from the offering were used to finance the acquisition of Genoptix. NeoGenomics Laboratories • 4

Future Integrating Genoptix and NeoGenomics into one company is a key focus in 2019. As we work through this integration, our utmost priority is service excellence, retention of our exceptional team, and achieving the highest levels of client satisfaction and retention. Strong service levels and the successful integration of Genoptix operations will allow us to grow our business, increase efficiency, and reduce cost per test. We are excited about the opportunities to be an even stronger company as a result of the Genoptix integration. Advances in science and medicine are continuing at a rapid rate, and innovation is as important as ever in our business. We will continue to maintain and expand our broad and innovative test menu which has helped make us a “one stop shop” for many clients who value sending all of their testing to one laboratory. We expect demand for our Pharma Services business to remain strong, and we believe high rates of growth for this business are sustainable. We also believe a natural synergy exists between our Clinical and Pharma divisions. Together, we are well positioned to support Pharma sponsors on clinical trials and companion diagnostic test development, and equally well positioned to rapidly offer new companion diagnostic tests as soon as a drug achieves regulatory approval. We expect our ability to provide companion diagnostic testing services will be increasingly important in the future. We are pleased to be a leader in Oncology diagnostics and believe that our services play a vital role in the health care system, and create value for patients, employees, customers and our investors. We are excited about the strength of our business and our opportunities in the near and long term. We believe NeoGenomics is competitively positioned to be the world’s leading cancer testing and information company as we look to the future. On behalf of our NeoGenomics team, thank you for your confidence in us and for your continued support of our Company. Sincerely, Douglas M. VanOort Chairman of the Board of Directors and Chief Executive Officer NeoGenomics Laboratories • 5

2019 Focus: CRITICAL SUCCESS FACTORS AND ACTIONS Employee Engagement Inclusion Actively seek the voice of our employees Demonstrate that we value our differences WRƓQGVROXWLRQVLQQRYDWHEXLOGRXU E\LQWHQWLRQDOO\IRVWHULQJDFXOWXUHRI World-Class FRPSDQ\DQGSURYLGHWLPHO\IHHGEDFN FROODERUDWLRQDQGLQFOXVLRQ Culture and recognition. Culture of Quality Process Improvement Incorporate FDA companion diagnostic- 6WUHDPOLQHDQGVLPSOLI\SURFHVVHVE\ Uncompromising related processes into Quality Management FRPELQLQJ*HQRSWL[ZLWK1(2 Quality System and achieve FDA readiness. Customer Experience 3URƓWDEOH*URZWK Exceptional Develop and launch innovative assays, 'ULYHSURƓWDEOHJURZWKZKLOH informatics products, research projects, VXFFHVVIXOO\LQWHJUDWLQJ*HQRSWL[DQG Service and and educational programs. PDLQWDLQLQJH[FHSWLRQDOVHUYLFHOHYHOV Growth NeoGenomics Laboratories • 6

Corporate Information Board of Directors &RUSRUDWH2IƓFHUV Medical Leadership Douglas M. VanOort Sharon A. Virag Lawrence M. Weiss, MD Chairman and CEO Chief Financial Officer Chief Scientific Officer Robert J. Shovlin Bruce K. Crowther Sally S. Agersborg, President, Clinical Services Director MD, PhD Alison L. Hannah George A. Cardoza Medical Director Director President, Pharma Services (Aliso Viejo, California) Raymond R. Hipp Jennifer M. Balliet Chief Culture Officer Josette William Ragheb, Director MD, PhD Bill Bonello Kevin C. Johnson Medical Director, Chief Strategy and Corporate Director Pharma Services Development Officer Steven C. Jones Director, Investor Relations Derek Lyle, MD Director Steven G. Brodie, Medical Director Stephen M. Kanovsky PhD, FACMG (Carlsbad, California/ Director Vice President of Worldwide Fort Myers, Florida) Pharma Services Operations Lynn A. Tetrault J. Christopher Mixon, MD Director Stephanie K. Bywater Chief Compliance Officer Medical Director (Nashville, Tennessee) Kathryn B. McKenzie Chief Accounting Officer David L. Morgan, MD Denise E. Pedulla Medical Director Corporate Secretary and (Houston, Texas) General Counsel Steven A. Ross Vice President, Chief Information Officer John S. Park Chief Marketing Officer &RUSRUDWH2IƓFHV Forward Looking Statements 12701 Commonwealth Drive, Suite 9 Fort Myers, FL 33913 Certain information contained in this annual report constitutes forward-looking statements for purposes of the safe harbor Transfer Agent provisions of The Private Securities Litigation Reform Act of 1995, Standard Registrar and Transfer Company 440 East 400 South, including the information set forth in the Letter to Shareholders. Suite 200, Salt Lake City, UT 84111 These forward-looking statements involve a number of risks Phone: 801.596.2150 and uncertainties that could cause actual future results to differ materially from those anticipated in the forward-looking Independent Registered Public Accounting Firm statements as the result of the Company’s ability to continue gaining new customers, offer new types of tests, integrate its Deloitte & Touche LLP acquisition of the Genoptix business, and otherwise implement New York, NY its business plan, as well as additional factors discussed under the Annual Meeting heading “Risk Factors” contained in Item 1A in our Annual Report on Form 10-K for the year ended December 31, 2018, filed with The annual meeting of stockholders will be held at the Hyatt the Securities and Exchange Commission on February 26, 2019. Coconut Point Resort at: 5001 Coconut Road Bonita Springs, All information in the Letter to Shareholders is as of release FL 34134 on June 6, 2019 at 10:00am EDT. and should not be relied upon as representing the Company’s estimates as of any subsequent date. While the Company may Stock Listing and Information elect to update forward-looking statements at some point in the The Company’s stock trades under the symbol “NEO” on the future, it specifically disclaims any obligation to do so, even if its NASDAQ Capital Market. estimates change.

NeoGenomics Laboratories is a specialized oncology reference laboratory providing the latest technologies, testing partnership opportunities, and interactive education to the oncology and pathology communities. We offer the complete VSHFWUXPRIGLDJQRVWLFVHUYLFHVLQPROHFXODUWHVWLQJ),6+F\WRJHQHWLFVŴRZF\WRPHWU\DQGLPPXQRKLVWRFKHPLVWU\ WKURXJKRXUQDWLRQZLGHQHWZRUNRI&$3DFFUHGLWHG&/,$FHUWLƓHGODERUDWRULHV Committed to research as the means to improve patient care, we provide Pharma Services for pharmaceutical companies, in vitro diagnostic manufacturers, and academic scientist-clinicians. We promote joint publications with our client physicians. NeoGenomics welcomes your inquiries for collaborations. Please contact us for more information. 12701 Commonwealth Dr., Suite 9 Fort Myers, FL 33913 Phone: 866.776.5907/ Fax: 239.690.4237 neogenomics.com © 2019 NeoGenomics Laboratories, Inc. All Rights Reserved. All other trademarks are the property of their respective owners. Rev. 041219