EX-99.1 ANNUAL REPORT
Published on April 17, 2020

Improving Patient Care 2019 Annual Report

Becoming the World’s Leading Cancer Testing and Information Company OUR COMMON PURPOSE We save lives by improving patient care. OUR VISION By providing uncompromising quality, exceptional service and innovative solutions, we are becoming the world’s leading cancer testing and information company. OUR VALUES Quality, Integrity, Accountability, Teamwork, and Innovation Key 2019 Strategic Accomplishments Key 2019 Financial Highlights • Substantially integrated Genoptix, acquired • Performed approximately 988,000 in December 2018. clinical oncology test for approximately • Completed a $160.8 million net equity 500,000 cancer patients. offering, and entered into a new $250 • Grew consolidated revenue growth million five-year Senior Secured Credit 47.7% year-over-year to $408.8 million. Agreement. • Increased Clinical Services test • Announced the building of a leading-edge volumes by 31.7% and average cancer diagnostics testing facility and new revenue per test by 13.3%. global headquarters in Fort Myers, Florida. • Grew Pharma Services revenue 36.7% • Added large national health system, Group to $47.7 million and increased backlog Purchasing Organization, and managed 31.8% to $130.3 million. care contracts. • Increased adjusted EBITDA by 31.4% • Significantly enhanced our next-generation to $57.2 million. sequencing capabilities. • Launched several key companion diagnostic tests. • Opened an oncology-focused clinical trials lab in Singapore. • Invested in future informatics capabilities.
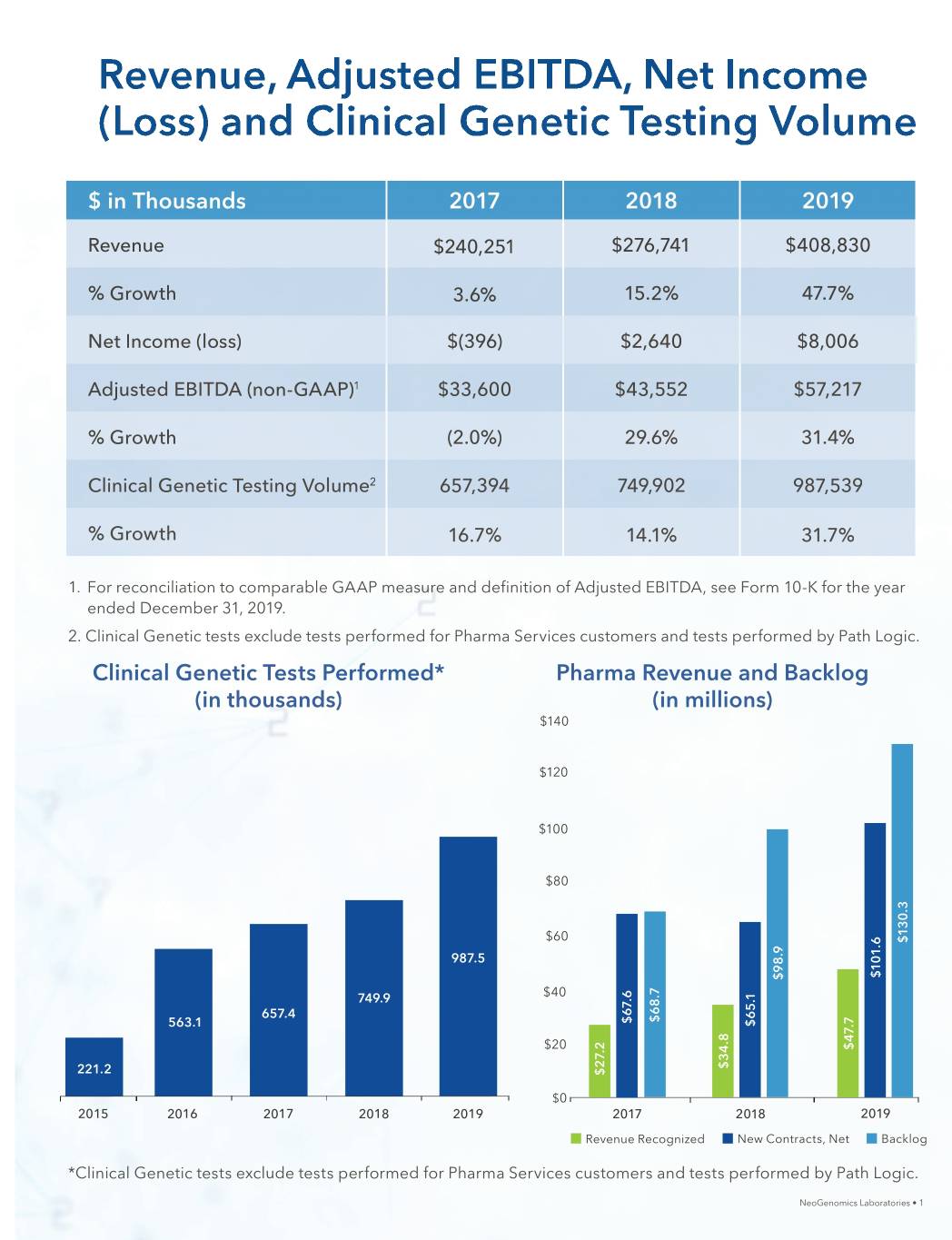
Revenue, Adjusted EBITDA, Net Income (Loss) and Clinical Genetic Testing Volume $ in Thousands 20172017 20182018 20192019 RevenueRevenue $240,251$240,251 $276,741$276,741 $408,830$408,830 % Growth 3.3.6%6% 15.2%15.2% 47.7%47.7% NetNet IncomeIncome (loss)(loss) $(396)$(396) $2,640$2,640 $8,006$8,006 AdjustedAdA justed EBITDA (n(non-GAAP)on-GGAAP)1 $33,600$333,600 $43,552$433,552 $57,217$557,217 % GrowthGrowth ( (2.0%)2.0%) 2 29.6%9.6% 31.4 31.4%% ClinicalClinical GenGeneticetic TesTestingting VolVolumeume2 657,657,39 3944 7 749,90249,902 987 987,539,539 % GrowthGrowth 16.7%16.77% 14.1%14.1% 31.7%31.7% 1. For rereconciliationconciliation to comparablecomparable GAAPGAAP measuremeassuru e and definitiondefinition ofof AdjustedAdjusted EBITDA,EBITDA, see FormForm 10-K forfor thethe year enendedded DecemberDecember 31,31, 2019.2019. 22.. ClinicalClinical GeGeneticnetic tests exexcludeclude tetestssts peperformedrfformed forfor Pharma SerServicesvices cucustomersstomers and tests performedperformed by PathPath Logic.Logic. ClinicalClinical GGeneticenetic TTestsests PPerformed*erformed* PPharmaharma RevenueRevenue aandnd BaBacklogcklog ((inin tthousands)housands) ((inin millions)millions) $140 $120 $100 $80 $60 $130.3 987.5 $101.6 $98.9 749.9 $40 657.4 $68.7 $67.6 563.1 $65.1 $20 $47.7 $34.8 221.2 $27.2 $0 2015 2016 2017 2018 2019 2017 2018 2019 Revenue Recognized New Contracts, Net Backlog **C*Clinicallinicaal GGeGeneticnetic tetestsests s exexcludeclude tetestssts s perfperformedormed forfor Pharma SServiceservices customcustomersers and teststests performed by Path Logic.Logic. NeoGenomicsNeoGenomics Laboratories • 1

Dear Fellow Shareholders: Just a few months ago, we could not have imagined the world being challenged by an unprecedented global health crisis. Governments, organizations, and individuals are coming together in extraordinary ways to ensure continuity of care for our most vulnerable populations — not only those impacted by the COVID-19 virus, but also those with other critical health conditions, including cancer. This crisis has also reminded us of the value that diagnostic testing plays in solving our most critical health care challenges. NeoGenomics’ employees work with enormous dedication to ensure that cancer patients have access to the most advanced diagnostic testing, even in a time of crisis. They work with a common purpose focused on patient care, and a set of values of Quality, Accountability, Integrity, Teamwork, and Innovation that define our culture. Their incredible effort and sustained commitment to excellence in oncology diagnostics makes us proud. While no company is immune from the economic impact of this current crisis, NeoGenomics is well-positioned to weather the storm. We exited 2019 with $173 million of cash and $131 million of availability on our credit facility, and maintain a strong balance sheet and significant liquidity today. Even as we weather the storm, we are maintaining focus on strengthening our competitive position over the long-term. I am highly confident in our employees and our culture, our competitive and financial strengths, and our long-term growth prospects. We are extremely excited about our outlook for NeoGenomics. We believe we have the essential strategic and operational building blocks in place to be a global leader in oncology diagnostics. And in this revolutionary time in oncology care, NeoGenomics’ competitive position and expertise is providing enormous opportunities for growth, and to save lives and improve cancer care. 2019 Highlights 2019 was an outstanding year for NeoGenomics. We grew revenue by nearly 50% to $409 million, including greater than 20% organic growth, and increased gross margin by more than 200 basis points year-over-year to 48.1%. More importantly, we significantly strengthened our competitive position as a leading global oncology diagnostic company. Clinical Services Division Our Clinical Services Division continued to drive growth across all testing modalities. Next Generation Sequencing and molecular testing were particularly strong and grew by approximately 50%. Even during an integration year, organic growth rates accelerated each quarter since we acquired Genoptix in December 2018. Clinical Division growth reflects a very high level of customer retention and satisfaction, and a steady increase in market share. Strategically, we continued to fortify our market position as the leading oncology-lab for hospitals and pathologists. We added large, national health system accounts, group purchasing contracts, and managed care contracts, and are now contracted with every major national payor and with most significant regional plans. We also greatly expanded our distribution channel and gained an excellent market position with community oncology practices through our acquisition of Genoptix. Technologically, we enhanced our next-generation-sequencing capabilities significantly, and are rapidly emerging as one of the largest providers of oncology-focused molecular testing in the country. Genoptix Acquisition We completed the sizable and strategically important acquisition of Genoptix on December 10, 2018. Since that time, our number one operating priority has been to successfully integrate the business, and we made significant strides in accomplishing that objective. Within seven weeks of closing the deal, we had fully integrated our two sales teams resulting in a doubling of the size of our team. We quickly integrated our medical staff and all functional areas. Over the course of the year, we made significant strides to realize some of the associated synergies from these activities. As I write this letter, we are in the final throes of integration activity — migrating former Genoptix customers to the same lab information and billing systems as legacy NeoGenomics customers. This migration opens the door to significant operating enhancements and cross-selling opportunities. In fact, we expect to see an improvement in margins in the second half of 2020 as we are able to focus on the type of cost and productivity improvement that has been a hallmark of our operations for years. 2 • NeoGenomics Laboratories

The Genoptix acquisition has already proven to be an important strategic initiative for our company. Our combination with Genoptix has set NeoGenomics apart from the rest of the industry with an unprecedented reach to all clinical customer segments, including hospitals, pathologists, and community oncology practices. Pharma Services Division Our Pharma Services Division supports pharmaceutical clients on a global basis across the drug development continuum, from research and development, through clinical trials testing, to commercialization of companion diagnostic tests. We currently provide service for more than 150 different clients around the world. In July we continued to expand our global offering, opening an oncology-focused clinical trials lab in Singapore. The Pharma business is stronger than it has ever been and is well-positioned to capitalize on a robust environment for oncology therapy development. In our Pharma Services Division, revenue increased 37% to $48 million. We signed more than $100 million of new contracts during the year and our backlog of signed contracts increased 32% to more than $130 million. With the recent acquisition of the Oncology Division assets of HLI, our backlog is approximately $145 million. In Pharma Services, we continue to benefit from our domain expertise, global expansion, investments in Flow Cytometry, global alliance with PPD, and positioning for companion diagnostics. Over the course of 2019, we assisted numerous pharmaceutical companies in securing FDA approvals for their therapies. People The ability to attract and retain talented individuals is a key competitive advantage for NeoGenomics — one that was put to the test in 2019 as we hired approximately 500 new employees to accommodate high-levels of growth, manage through the Genoptix acquisition, and prepare for future growth initiatives. Importantly, we maintained our typically high levels of employee retention, even as we accommodated this substantial growth. Strengthened Financial Position We also continued to strengthen our Balance Sheet in 2019. In May, we completed a public offering of 8 million shares of common stock realizing net proceeds of approximately $161 million. In June, we entered into a new $250 million five-year Senior Secured Credit Agreement consisting of a $100 million Revolving Credit Facility, a $100 million initial Term Loan and a $50 million Delayed Draw Term Loan which is available for 18 months. We exited 2019 with a strong balance sheet, and approximately $131 million of available borrowing capacity on our credit facilities. After year end, we acquired the Oncology Division assets of Human Longevity for approximately $37 million. State-of-the-Art Oncology Laboratory and Global Headquarters In July, we announced our plan to build a leading-edge cancer diagnostics testing facility and new global business headquarters in Fort Myers, Florida. The new facility is expected to open in 2021. The 150,000-square-foot laboratory and headquarters facility (with space for additional expansion if needed) will incorporate innovative technology to deliver comprehensive oncology testing for physicians and their patients spanning all available testing modalities including a state- of-the-art molecular lab with next generation sequencing capabilities. Strategic Priorities for 2020 and Beyond Each year we create a company-wide FOCUS document to summarize and articulate our near-term strategic and operating priorities. This document is a tool that is used by the entire company to ensure alignment, focus, and continuous improvement. Our 2020 FOCUS theme, “Improving Patient Care”, encapsulates our common purpose. To support this purpose, we will pursue initiatives to maintain and advance world-class culture, deliver uncompromising quality and exceptional service and drive innovation and profitable growth. A summary of the 2020 FOCUS document is included in this Annual Report. NeoGenomics Laboratories • 3

World-Class Culture Enhancing our culture, and keeping it closely aligned with the values of our Company, is a key priority. We are investing in the development of our people by creating mentoring, coaching and training opportunities to enhance and capitalize on the talent within our Company. These initiatives are designed to foster a culture of accountability and empowerment and to ensure the success of our Company. We are actively promoting the health and well-being of our employees. We recognize that health goes beyond health benefits and preventative care, and includes the quality of our physical work environment and programs that encourage social responsibility and community engagement. Inclusive communication is a key element in our high performance culture. Effective communication facilitates collaboration and enhances our employees’ understanding of their contributions to the Company’s overall objectives. We work hard to foster employee engagement through collaborative forums, frequent team dialogue and recognition programs to reward teams for exceptional performance. Continually strengthening and developing a world-class culture is clearly a strategic priority for us, enabling outstanding rates of employee engagement and an ability to recruit and retain world-class talent. Uncompromising Quality and Exceptional Service Maintaining the highest quality laboratory operations and service levels has enabled us to consistently grow our business. We continuously look for ways to improve quality and implement best practices to streamline processes. We focus on automation to improve efficiency in operations. We invest in quality systems and processes and strive for the highest standards of performance for our clinical clients and for pharmaceutical customers. Maintaining consistently high quality is a strategic imperative that sustains our long-term competitive position. Innovation and Growth We have a broad and deep distribution system in oncology, which is fueled by our comprehensive oncology test menu. We are building on this strategically important competitive attribute by introducing new tests and services to help our customers deliver better care for cancer patients. Our current focus is to expand on our strength in Next Generation Sequencing, fortify our leading position in companion diagnostics, and build an informatics division that will bring data-driven solutions to help key stakeholders solve real-world problems. Each of these areas is rich in opportunity for growth. Next Generation Sequencing (NGS) Next Generation Sequencing is an area of particular development focus for us, and we expect to expand our high-quality product offering in 2020 to include a suite of liquid biopsy products for cases where tissue samples are not possible to obtain. We are working to expand our offering of RNA-based sequencing assays for both solid tumor and hematologic malignancies, and on new assays for identifying minimal residual disease, particularly for hematologic neoplasms. Because of our reach into thousands of hospitals and oncology practices, we are able to facilitate the adoption of these advanced oncology diagnostic tools beyond the academic environment into the community setting so that cancer patients can have access to the leading oncology care in their own communities. Importantly, our team of over 120 MDs and PhDs, along with the highly-trained oncology-focused sales team, provides continuous education to our clients to ensure that they remain abreast of developments in oncology. We made an important strategic move to build our NGS Pharma Services product capabilities in January 2020 by acquiring the Oncology Division assets of Human Longevity, Inc. The team, based in La Jolla, California, performs Next Generation Sequencing services for pharmaceutical customers, including germline, whole exome and whole genome sequencing. This acquisition brings an even more experienced, specialized molecular workforce with strong Next Generation Sequencing expertise, particularly in serving pharmaceutical customers. This business generated approximately $10 million in revenues in 2019 and ended the year with a backlog of approximately $15 million of signed contracts. 4 • NeoGenomics Laboratories

Companion Diagnostics Companion Diagnostics is an important area of growth as precision medicine and immuno-therapies increasingly rely on these tests to predict their effectiveness in patients. Included in our backlog of signed contracts are approximately 30 different companion diagnostic projects for a variety of Pharma and Biotech companies. We are increasingly in discussions with Pharma sponsors to help with their companion diagnostic projects. We also have agreements with several large pharmaceutical companies to provide “Day-1” commercial launch services, and advanced analytical support, for companion diagnostic testing associated with drugs in the late-stage pipeline. Few labs have our same ability to take an oncology companion test across the continuum from development, through clinical trials, and into the market. Our leading position in companion diagnostics was demonstrated in 2019 when we announced that we would serve as the Novartis “Day 1” Preferred Laboratory Partner for PIK3CA testing. The PIK3CA assay is a companion diagnostic test approved by the FDA to aid clinicians in identifying breast cancer patients suitable for treatment with Piqray® (alpelisib), a newly approved therapy developed and marketed by Novartis. Informatics In 2019, we began to invest in a new data and informatics business. This important capability leverages our unique market position and oncology expertise to help our stakeholders solve real-world problems such as identifying patients for clinical trials or implementing clinical decision support tools for physicians, health care systems, payors, pharma companies, and patients. While we are in the very early innings in terms of product development, we already have significant engagement from various stakeholders including global pharmaceutical firms, large national health systems and major managed care payors. We are serious about our Informatics initiative, and currently have nearly 30 employees devoted to this strategic investment initiative. We expect this new Division to be an incremental source of revenue in the long-term, while strengthening our competitive position in both the Clinical and Pharma Services Divisions. Summary NeoGenomics is truly a unique company. We are intensely focused on improving and saving the lives of cancer patients, are driven by an uncompromising set of values, and are excited to provide innovative and leading-edge technologies for our many stakeholders. We are fortunate to have investors who believe in us and support our business and our strategies. We don’t take any of that for granted. Sincerely, Douglas M. VanOort Chairman of the Board of Directors and Chief Executive Officer NeoGenomics Laboratories • 5
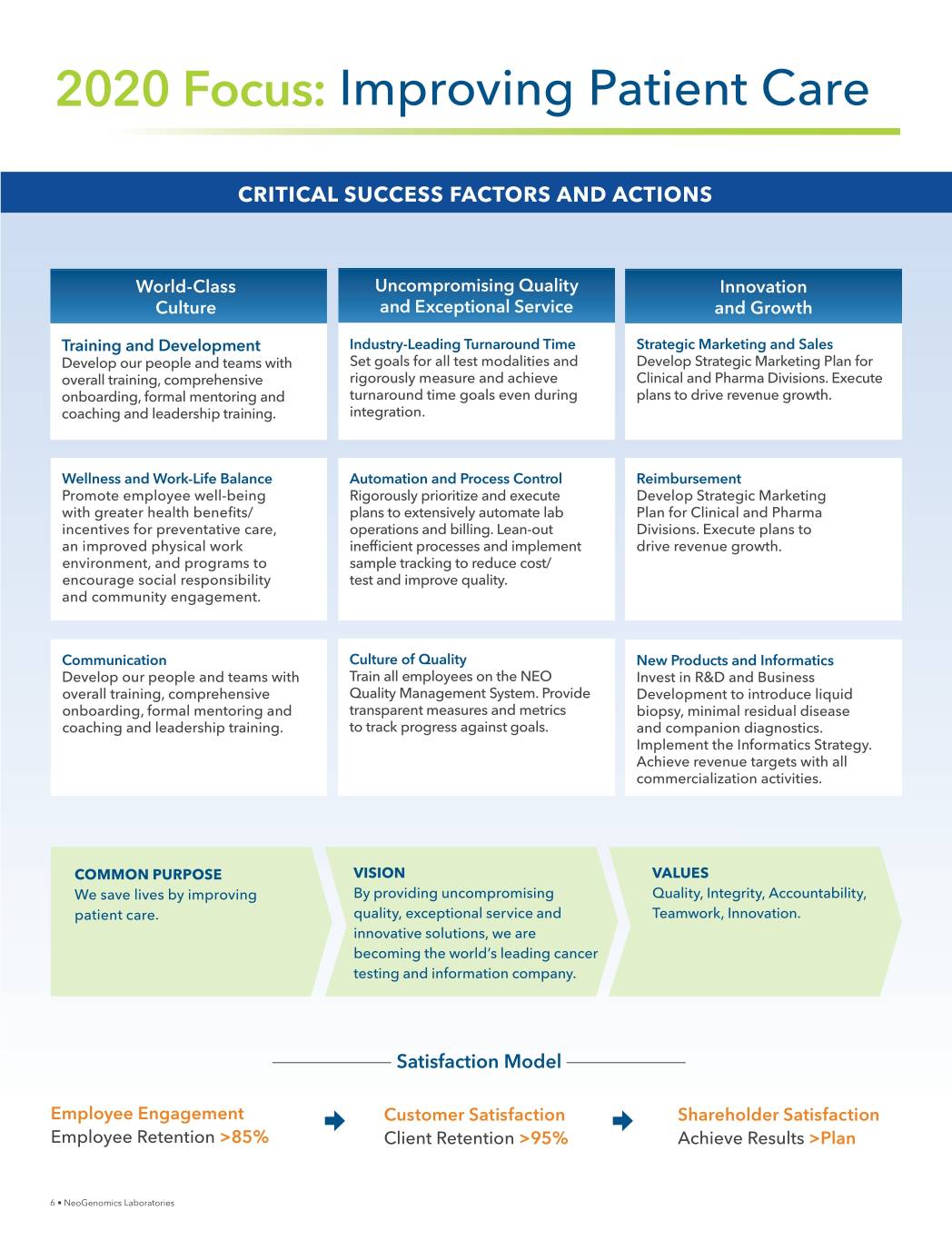
2020 Focus: Improving Patient Care CRITICAL SUCCESS FACTORS AND ACTIONS World-Class Uncompromising Quality Innovation Culture and Exceptional Service and Growth Training and Development Industry-Leading Turnaround Time Strategic Marketing and Sales Develop our people and teams with Set goals for all test modalities and Develop Strategic Marketing Plan for overall training, comprehensive rigorously measure and achieve Clinical and Pharma Divisions. Execute onboarding, formal mentoring and turnaround time goals even during plans to drive revenue growth. coaching and leadership training. integration. Wellness and Work-Life Balance Automation and Process Control Reimbursement Promote employee well-being Rigorously prioritize and execute Develop Strategic Marketing ZLWKJUHDWHUKHDOWKEHQHƓWV plans to extensively automate lab Plan for Clinical and Pharma incentives for preventative care, operations and billing. Lean-out Divisions. Execute plans to an improved physical work LQHIƓFLHQWSURFHVVHVDQGLPSOHPHQW drive revenue growth. environment, and programs to VDPSOHWUDFNLQJWRUHGXFHFRVW encourage social responsibility test and improve quality. and community engagement. Communication Culture of Quality New Products and Informatics Develop our people and teams with Train all employees on the NEO Invest in R&D and Business overall training, comprehensive Quality Management System. Provide Development to introduce liquid onboarding, formal mentoring and transparent measures and metrics biopsy, minimal residual disease coaching and leadership training. to track progress against goals. and companion diagnostics. Implement the Informatics Strategy. Achieve revenue targets with all commercialization activities. COMMON PURPOSE VISION VALUES We save lives by improving By providing uncompromising Quality, Integrity, Accountability, patient care. quality, exceptional service and Teamwork, Innovation. innovative solutions, we are becoming the world’s leading cancer testing and information company. Satisfaction Model Employee Engagement Customer Satisfaction Shareholder Satisfaction Employee Retention >85% Client Retention >95% Achieve Results >Plan 6 • NeoGenomics Laboratories

Corporate Information Board of Directors &RUSRUDWH2IƓFHUV Medical Leadership Douglas M. VanOort Kathryn B. McKenzie Lawrence M. Weiss, M.D. Chairman and CEO Chief Financial Officer Chief Medical Officer Bruce K. Crowther Denise E. Pedulla Sally S. Agersborg, M.D., Ph.D. Director General Counsel and Corporate Secretary Medical Director, Alison L. Hannah, M.D. Aliso Viejo, California Robert J. Shovlin Director President, Clinical Services Derek D. Lyle, M.D. Raymond R. Hipp Medical Director, Carlsbad, George A. Cardoza California and Director President, Pharma Services Fort Myers, Florida Kevin C. Johnson William B. Bonello J. Christopher Mixon, M.D. Director President, Informatics and Laboratory Director, Director, Investor Relations Nashville, Tennessee Steven C. Jones Director Douglas M. Brown David L. Morgan, M.D. Chief Strategy and Corporate Laboratory Director, Stephen M. Kanovsky Development Officer Houston, Texas Director Jennifer M. Balliet Josette William Ragheb, Lynn A. Tetrault Chief Culture Officer M.D., Ph.D. Global Medical Director, Director Stephanie K. Bywater Pharma Services Chief Compliance Officer Steven A. Ross Vice President, Chief Information Officer &RUSRUDWH2IƓFHV Forward Looking Statements 12701 Commonwealth Drive, Suite 9 Fort Myers, FL 33913 Certain information contained in this annual report constitutes forward-looking statements for purposes of the safe harbor Transfer Agent provisions of The Private Securities Litigation Reform Act of 1995, Standard Registrar and Transfer Company 440 East 400 South, including the information set forth in the Letter to Shareholders. Suite 200, Salt Lake City, UT 84111 These forward-looking statements involve a number of risks Phone: 801.596.2150 and uncertainties that could cause actual future results to differ materially from those anticipated in the forward-looking Independent Registered Public Accounting Firm statements as the result of the Company’s ability to continue gaining new customers, offer new types of tests, integrate its Deloitte & Touche LLP New York, NY acquisition of the Genoptix business, and otherwise implement Annual Meeting its business plan, as well as additional factors discussed under the heading “Risk Factors” contained in Item 1A in our Annual Report Our annual meeting will be a completely virtual meeting of on Form 10-K for the year ended December 31, 2019, filed with stockholders on May 28, 2020 at 10:00 a.m. Eastern Time. the Securities and Exchange Commission on February 28, 2020. www.virtualshareholdermeeting.com/NEO2020 All information in the Letter to Shareholders is as of release and should not be relied upon as representing the Company’s Stock Listing and Information estimates as of any subsequent date. While the Company may elect to update forward-looking statements at some point in the The Company’s stock trades under the symbol “NEO” on the future, it specifically disclaims any obligation to do so, even if its NASDAQ Capital Market. estimates change.

NeoGenomics Laboratories is a specialized oncology reference laboratory providing the latest technologies, testing, partnership opportunities, and interactive education to the oncology and pathology communities. We offer the complete VSHFWUXPRIGLDJQRVWLFVHUYLFHVLQPROHFXODUWHVWLQJ),6+F\WRJHQHWLFVŴRZF\WRPHWU\DQGLPPXQRKLVWRFKHPLVWU\ WKURXJKRXUQDWLRQZLGHQHWZRUNRI&$3DFFUHGLWHG&/,$FHUWLƓHGODERUDWRULHV &RPPLWWHGWRUHVHDUFKDVWKHPHDQVWRLPSURYHSDWLHQWFDUHZHSURYLGH3KDUPD6HUYLFHVIRUSKDUPDFHXWLFDO FRPSDQLHVLQYLWURGLDJQRVWLFPDQXIDFWXUHUVDQGDFDGHPLFVFLHQWLVWFOLQLFLDQV:HSURPRWHMRLQWSXEOLFDWLRQVZLWKRXU FOLHQWSK\VLFLDQV1HR*HQRPLFVZHOFRPHV\RXULQTXLULHVIRUFROODERUDWLRQV3OHDVHFRQWDFWXVIRUPRUHLQIRUPDWLRQ 12701 Commonwealth Dr., Suite 9 Fort Myers, FL 33913 Phone: 866.776.5907/ Fax: 239.690.4237 neogenomics.com © 2020 NeoGenomics Laboratories, Inc. All Rights Reserved. $OORWKHUWUDGHPDUNVDUHWKHSURSHUW\RIWKHLUUHVSHFWLYHRZQHUV Rev. 040220