EX-99.1
Published on April 14, 2022

NeoGenomics Laboratories — II Transforming Patient Care 2021 ANNUAL REPORT I — NeoGenomics Laboratories NeoGenomics Laboratories is a speciali d oncolo eference laboratory providing the latest technologies, testing, partnership opportunities, and interactive education to o logy and pathology communities. We offer the complete spectrum of diagnostic services in molecular testing, FISH, togenetics, flow cytometry, immunohistochemistry, and morphologic analysis through our world-wide network of CAP-accredited, CLIA-certified laboratories. Committed to research as the means to improve patient care, we provide Pharma Services for pharmaceutical companies, in vitro diagnostic manufacturers, and academic scientist-clinicians. We promote joint publications with our client physicians. NeoGenomics welcomes your inquiries for collaborations. Please contact us for more information. 9490 NeoGenomics Way Fort Myers, FL 33912 Phone: 866.776.5907 www.neogenomics.com © 2022 NeoGenomics Laboratories, Inc. All rights reserved. All trademarks are the property of their respective owners. Rev. 040622 Dze gy r the nco cy Exhibit 99.1

Key 2021 Financial Highlights and Accomplishm nts • Increased consolidated venue by 9% y r-over-year. • Clinical Services revenue in ased 6 to $404 million. • Grew Pharma Services revenue 2 % to $80 million and increased backlog to $267 million. • Strengthened financial position with $743 million net convertible note and equity offerings. • Acquired Trapelo Health and Inivata Limited. • The Inivata acquisition added liquid biopsy platform technology, including MRD testing capabilities, to our comprehensive portfolio of oncology testing solutions. • Transitioned the majority of our testing in Fort Myers to our new headquarters and laboratory facility, providing significant additional capacity for future growth. OUR COMMON PURPOSE We save lives by improving patient care. OUR VISION By providing uncompromising quality, exceptional service and innovative solutions, we are becoming the world’s leading cancer testing nd i ormation company. OUR VALUES Quality, Integrity, Accountabil y Teamwork, and Innova n Becoming the World’s Leading Cancer Testing and Information Company Dere ea cre % 9 r tio fa it , tnf Corporate Officers William B. Bonello Chief Financial Officer Halley E. Gilbert Chief Legal Officer David B. Sholehvar, M.D. President, Clinical Services Gina M. Wallar President, Pharma Services Clive D. Morris, M.D. President, Inivata Jennifer M. Ballie Chief Culture Officer Douglas M. Brown Chief Strategy and Corporate Development O Cynthia J Dieter Chief Accou ng Offi Kath n B. McK nzie Chief Sus inability and sk Offi J n P. Mooney Chief T nology Officer Marcus Silva C ef Marketing Officer utan Hashemi C ef Compliance Officer Board of Directors Lynn A. Tetrault Executive Chair Bruce K. Crowther Director David J. Daly Director Alison L. Hannah, M.D. Director Stephen M. Kanovsky Director Michael A. Kelly Director Rachel A. Stahler Director Medical Leadership Shashikant Kulkarni, M.S., Ph.D., MBA, FACMG Executive Vice President, Research and Development, Chief Scientific Officer Sally S. Agersborg, M.D., Ph.D. Chief Medical Officer, Laboratory Operations Derek Lyle, M.D. Chief Medical Officer, Clinical Services Josette William Ragheb, M.D., Ph.D. G al Medical Director, Pharma Services Vladislav Chizhevsky, M.D. Medical Director Aliso Viejo, California Anahit Nowrouzi, M.D. Medical Director Fort Myers, Florida Tricia Peters, M.D. Medical Director Houston, Texas J. Christopher Mixon, M.D. Medical Director Nashville, Tennessee Corporate Offices 9490 NeoGenomics Way Fort Myers, Florida 33912 Independent Registered Public Accounting Firm Deloitte & Touche LLP San Diego, CA Annual Meeting Our annual meeting will be a virtual meeting of the stockholders on June 2, 2022 at 10:00 a.m. Eastern Time. www.virtualshareholdermeeting.com/NEO2022 Stock Listing and Information The Company’s stock trades under the symbol “NEO” on The Nasdaq Stock Market LLC. Forward-Looking Statements Certain information contained in this annual report constitutes forward- looking statements for purposes of safe harbor provisions of The Private Securities Litigation Reform Act of 1995, including the information set forth in the Letter to the Shareholders. These forward-looking statements involve a number of risks and uncertainties that could cause actual future results to differ materially from those anticipated in the forward-looking statements as the result of the Company’s ability to continue gaining new customers, offer new types of tests and otherwise integrate its business plan, as well as additional factors discussed under the heading “Risk Factors” contained in Item 1A in our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission on February 25, 2022. All information in the Letter to the Shareholders is as of release and should not be relied upon as representing the Company’s estimates as of any subsequent date. While the Company may elect to update forward-looking statements at some point in the future, it specifically disclaims any obligation to do so, even if its estimates change. Corporate Information hi H hi ry ta oh ech B. afficer. nti cer e Ri cer ft tlob
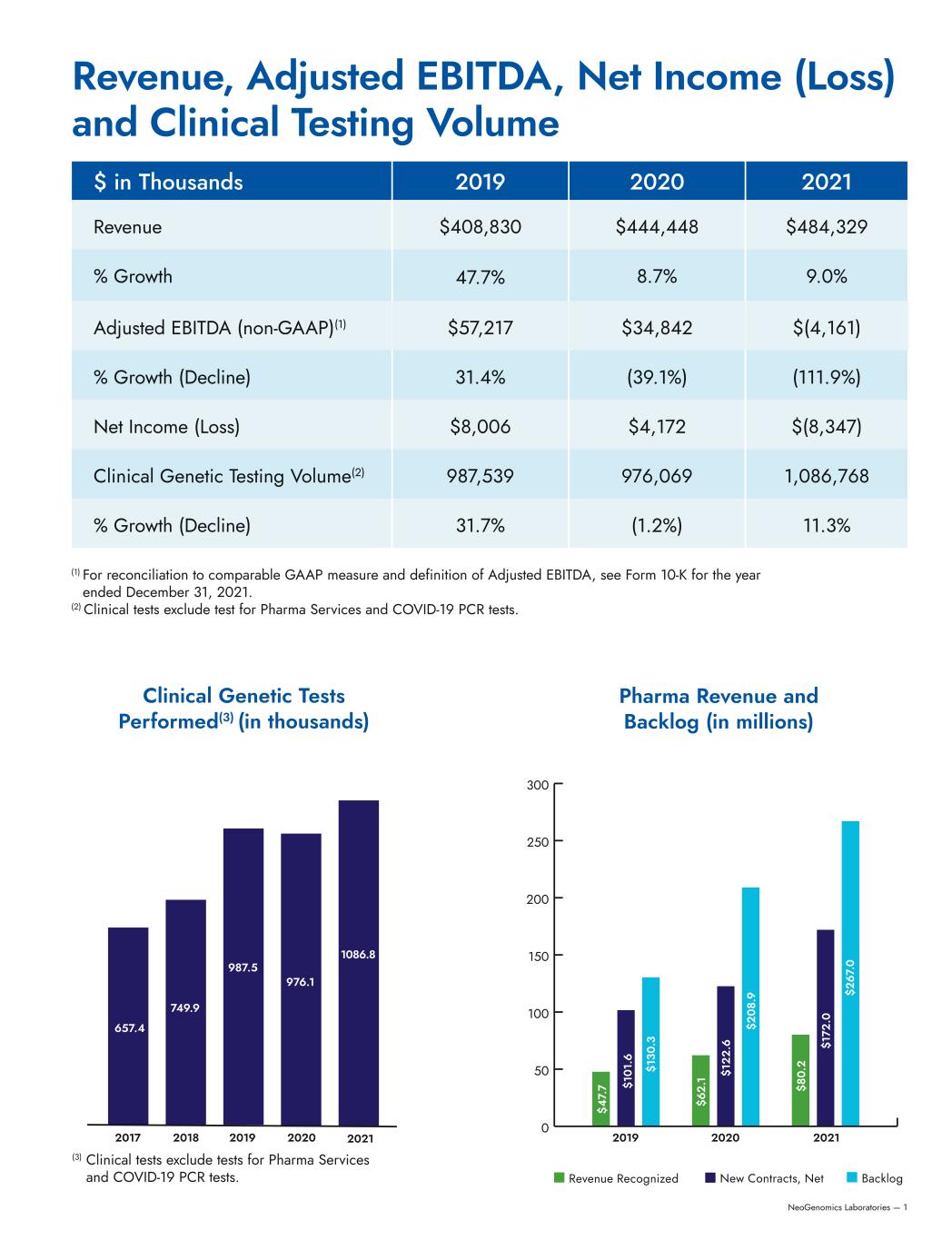
NeoGenomics Laboratories — 1 Clinical Genetic Tests Performed(3) (in thousands) Pharma Revenue and Backlog (in millions) $ in Thousands 2019 2020 2021 Revenue $408,830 $444,448 $484,329 % Growth 47.7% 8.7% 9.0% Adjusted EBITDA (non-GAAP)(1) $57,217 $34,842 $(4,161) % Growth (Decline) 31.4% (39.1%) (111.9%) Net Income (Loss) $8,006 $4,172 $(8,347) Clinical Genetic Testing Volume(2) 987,539 976,069 1,086,768 % Growth (Decline) 31.7% (1.2%) 11.3% (1) For reconciliation to comparable GAAP measure and definition of Adjusted EBITDA, see Form 10-K for the year ended December 31, 2021. (2) Clinical tests exclude test for Pharma Services and COVID-19 PCR tests. Revenue, Adjusted EBITDA, Net Income (Loss) and Clinical Testing Volume Revenue Recognized New Contracts, Net Backlog (3) Clinical tests exclude tests for Pharma Services and COVID-19 PCR tests. 657.4 749.9 987.5 976.1 1086.8 2017 2019 2020 20212018 2019 2020 2021 0 50 100 150 200 250 300 $4 7. 7 $6 2. 1 $8 0. 2 $1 01 .6 $1 22 .6 $1 72 .0 $1 30 .3 $2 08 .9 $2 67 .0

2 — NeoGenomics Laboratories Dear Fellow Shareholders: 2021 was a year of investment to fortify our position as a leading cancer testing and information company and to better position NeoGenomics for long-term growth. In June, we completed the acquisition of Inivata Limited (“Inivata”), positioning NeoGenomics to be a leading player in the large and rapidly growing market for minimal residual disease testing. In April, we completed the acquisition of Trapelo Health (“Trapelo”), an oncology-focused healthcare information company with a proprietary clinical decision support system. In 2021 we also opened a new laboratory in Suzhou, China, rounding out the global footprint for our Pharma Services business, and at year end we opened our new and expanded laboratory and global headquarters in Fort Myers, Florida. 2021 was also a year in which we managed through some significant changes and challenges. The emergence of the Delta and Omicron COVID-19 variants had an adverse impact on revenue and created temporary staffing and supply shortages. We are proud of the way that our employees came together as a team to overcome these situations, always with an eye to the physicians and patients whom we serve. We had changes in our leadership team, with the retirement of our long-time Chairman and Chief Executive Officer Doug VanOort and a handful of other executives. While the transition did create some disruption to our operations, we are proud that we remained steadfastly focused on our mission and vision throughout the year. In the midst of these changes, our teams worked tirelessly to continue delivering high-quality diagnostic testing for physicians and cancer patients to help them with their cancer journey. Certainly, our work is having a positive impact for patients. During the year, we performed 1.1 million tests for over 500,000 patients and provided critical services on over 1,000 projects for Pharma sponsors as they developed exciting new cancer therapies. I am incredibly proud of the hard work and dedication of each of our over 2,000 employees and contracted pathologists for their contributions to NeoGenomics, our clients and their patients. Strategic Growth Following our 2020 strategic collaboration and minority investment in Inivata, a leader in liquid biopsy testing and minimal residual disease (“MRD”) technology, we completed the acquisition of Inivata in 2021. This is allowing us to combine Inivata’s innovative technology with our leading community oncology platform. In addition to the InVisionFirst®-Lung liquid biopsy assay, Inivata brings a highly sensitive RaDaR™ liquid biopsy assay, targeting the emerging and significant opportunity for MRD testing. Already in 2022, we announced that Inivata received the CE mark for its RaDaR™ assay to detect MRD and recurrence. We are excited about the addition of this important emerging technology to NeoGenomics’ long list of oncology diagnostic capabilities. Informatics Our Informatics capabilities are also growing. In 2021, we completed the acquisition of Trapelo, an information technology company focused on precision oncology. Trapelo offers a first-of-its-kind, interoperable, decision-support platform for physicians, laboratories, and payers to inform testing and treatment selection, improve clinical trials matching, streamline workflow and facilitate real-time alignment with the most current clinical evidence. The platform improves patient access

NeoGenomics Laboratories — 3 to medically appropriate and high-quality treatment options. The Trapelo team has made great progress scaling next- generation clinical decision support for oncologists, and we anticipate launching a web-based “quickstart” version of the tool by the middle of 2022. Biomarker Collaborative We are also excited about harnessing information to benefit patients directly. In January 2022 we announced a groundbreaking partnership with Biomarker Collaborative to help cancer patients who test positive for specific biomarkers to instantly connect with support groups and peers. Global Headquarters We added capacity to support future growth by opening doors to our new state-of-the-art oncology laboratory and global headquarters in Fort Myers, Florida and we began moving operations in the fourth quarter. This new 150,000 square foot facility incorporates innovative technology, triples our lab capacity in Fort Myers, and is a new “front door” for our dedicated NeoGenomics teams worldwide. The new facility is providing exciting new growth opportunities for our company, and for the communities in Southwest Florida. Pharma Services Our Pharma Services capabilities are expanding on a worldwide scale. At the request of our Pharma clients, we opened our third Pharma Services lab outside the U.S. in Suzhou, China to support oncology drug development worldwide. Quality Strategic growth initiatives include investments demonstrating our progress along our Quality journey. We are proud to have achieved multiple lab accreditations, including the new Fort Myers laboratory, and ISO 15189 accreditation for two of our international sites, Singapore and Rolle, Switzerland. 2021 Highlights Despite challenges, consolidated revenue in 2021 increased 16% from last year when excluding COVID-19 PCR testing revenue for both years. Clinical volume growth was stronger in the front part of the year, but Delta and Omicron COVID-19 variants pressured volume growth in the back half of the year. Our Clinical Services operations teams made tremendous progress improving service levels and driving down backlogs of overdue tests caused by employee shortages and supply-chain disruptions. Demand for our Pharma Services remained strong despite the pandemic, as we booked approximately $172 million in net newly-signed contracts and ended the year with backlog of approximately $267 million.

4 — NeoGenomics Laboratories Clinical testing services continue to be the backbone of the company and represented 83% of our revenues during the year. Our average customer account in 2021 ordered more than 250 tests during the year, and many of our customers view us as their only or primary reference lab for cancer testing. A recent national survey of independent labs and hospitals named NeoGenomics the number one preferred reference lab for cancer testing by pathologists with a 25% market share. Our latest Net Promoter Score Survey yielded a 62, continuing our track record of delivering elite service levels to our customers. We are proud of the quality of the service that our teams continued to deliver during the year. Our People Our people and our culture are key to our success. During the year we continued to make improvements in our workplace and invested in ongoing opportunities for employee development in a diverse and inclusive environment. We have worked over many years to reflect gender and ethnic diversity and inclusion on our Board, and diversity in gender and ethnicity is well-established within our workforce. As of December 31, 2021, women made up 59% of our global workforce. Women represented 53% of our supervisory or higher positions, 44% of our executive team and vice presidents, and 33% of our Board of Directors. Ethnicity is also strongly represented: 52% of our workforce and 11% of our Board of Directors were racially or ethnically diverse. Diversity is an active conversation at NeoGenomics including through employee-initiated and employee-led employee resource groups (“ERGs”) such as LGBTQ@Neo, Women@Neo, Veterans@Neo, We S.T.A.N.D@Neo (Standing Together Against Negativity and Discrimination), and Wellness@Neo. These ERGs reinforce our commitment to diversity by fostering community, providing education and support across the business, and facilitating dialogue on relevant and critical employee topics. We made strong additions to our leadership team recently by recruiting several talented executives, including Dr. Shashi Kulkarni, Dr. David Sholehvar and Hutan Hashemi. Dr. Shashi Kulkarni, a world-renowned expert and key opinion leader in cancer genomics, joined as Executive Vice President for Research and Development and Chief Scientific Officer; Dr. David Sholehvar, a seasoned leader in the diagnostics industry, joined as Clinical Services Division President; and Hutan Hashemi returned to NeoGenomics as Chief Compliance Officer, bringing notable legal and compliance experience to our teams. Each of these individuals is passionate and committed to leading NeoGenomics to new heights, and I am confident they will. CEO Transition In March 2022 we announced that Mark Mallon would step down as Chief Executive Officer (“CEO”) and a member of the Board. As part of the transition, I assumed the role of Executive Chair while we search for a new CEO. We also established an Interim Office of the CEO, comprised of Bill Bonello, our Chief Financial Officer, Jennifer Balliet, our Chief Culture Officer, and Doug Brown, our Chief Strategy and Business Development Officer, to provide leadership continuity and seamless operational management during the transition. Dear Fellow Shareholders (cont.)

NeoGenomics Laboratories — 5 I joined the Board of this company 7 years ago because I was inspired by NeoGenomics’ mission, vision, and values and the Board and executive management team remain fully committed to them. NeoGenomics’ people, culture, values, and the difference that we make for patients each and every day differentiates us in the marketplace and provides value to our stakeholders. As we look forward, we are committed to continuing to deliver exceptional quality and service, guided by an unwavering set of values and a mission more significant than any individual. Summary NeoGenomics remains a truly unique company. We are a team of dedicated people who want to make a difference in the lives of patients each and every day. We are also excited to provide innovative and leading-edge technologies for our many stakeholders. We are fortunate to have investors who believe in us and support our business and our strategies. Thank you for your continued support. Warmest Regards, Lynn Tetrault Executive Chair of the Board

Key 2021 Financial Highlights and Accomplishm nts • Increased consolidated venue by 9% y r-over-year. • Clinical Services revenue in ased 6 to $404 million. • Grew Pharma Services revenue 2 % to $80 million and increased backlog to $267 million. • Strengthened financial position with $743 million net convertible note and equity offerings. • Acquired Trapelo Health and Inivata Limited. • The Inivata acquisition added liquid biopsy platform technology, including MRD testing capabilities, to our comprehensive portfolio of oncology testing solutions. • Transitioned the majority of our testing in Fort Myers to our new headquarters and laboratory facility, providing significant additional capacity for future growth. OUR COMMON PURPOSE We save lives by improving patient care. OUR VISION By providing uncompromising quality, exceptional service and innovative solutions, we are becoming the world’s leading cancer testing nd i ormation company. OUR VALUES Quality, Integrity, Accountabil y Teamwork, and Innova n Becoming the World’s Leading Cancer Testing and Information Company Dere ea cre % 9 r tio fa it , tnf Corporate Officers William B. Bonello Chief Financial Officer Halley E. Gilbert Chief Legal Officer David B. Sholehvar, M.D. President, Clinical Services Gina M. Wallar President, Pharma Services Clive D. Morris, M.D. President, Inivata Jennifer M. Ballie Chief Culture Officer Douglas M. Brown Chief Strategy and Corporate Development O Cynthia J Dieter Chief Accou ng Offi Kath n B. McK nzie Chief Sus inability and sk Offi J n P. Mooney Chief T nology Officer Marcus Silva C ef Marketing Officer utan Hashemi C ef Compliance Officer Board of Directors Lynn A. Tetrault Executive Chair Bruce K. Crowther Director David J. Daly Director Alison L. Hannah, M.D. Director Stephen M. Kanovsky Director Michael A. Kelly Director Rachel A. Stahler Director Medical Leadership Shashikant Kulkarni, M.S., Ph.D., MBA, FACMG Executive Vice President, Research and Development, Chief Scientific Officer Sally S. Agersborg, M.D., Ph.D. Chief Medical Officer, Laboratory Operations Derek Lyle, M.D. Chief Medical Officer, Clinical Services Josette William Ragheb, M.D., Ph.D. G al Medical Director, Pharma Services Vladislav Chizhevsky, M.D. Medical Director Aliso Viejo, California Anahit Nowrouzi, M.D. Medical Director Fort Myers, Florida Tricia Peters, M.D. Medical Director Houston, Texas J. Christopher Mixon, M.D. Medical Director Nashville, Tennessee Corporate Offices 9490 NeoGenomics Way Fort Myers, Florida 33912 Independent Registered Public Accounting Firm Deloitte & Touche LLP San Diego, CA Annual Meeting Our annual meeting will be a virtual meeting of the stockholders on June 2, 2022 at 10:00 a.m. Eastern Time. www.virtualshareholdermeeting.com/NEO2022 Stock Listing and Information The Company’s stock trades under the symbol “NEO” on The Nasdaq Stock Market LLC. Forward-Looking Statements Certain information contained in this annual report constitutes forward- looking statements for purposes of safe harbor provisions of The Private Securities Litigation Reform Act of 1995, including the information set forth in the Letter to the Shareholders. These forward-looking statements involve a number of risks and uncertainties that could cause actual future results to differ materially from those anticipated in the forward-looking statements as the result of the Company’s ability to continue gaining new customers, offer new types of tests and otherwise integrate its business plan, as well as additional factors discussed under the heading “Risk Factors” contained in Item 1A in our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the Securities and Exchange Commission on February 25, 2022. All information in the Letter to the Shareholders is as of release and should not be relied upon as representing the Company’s estimates as of any subsequent date. While the Company may elect to update forward-looking statements at some point in the future, it specifically disclaims any obligation to do so, even if its estimates change. Corporate Information hi H hi ry ta oh ech B. afficer. nti cer e Ri cer ft tlob

NeoGenomics Laboratories — II Transforming Patient Care 2021 ANNUAL REPORT I — NeoGenomics Laboratories NeoGenomics Laboratories is a speciali d oncolo eference laboratory providing the latest technologies, testing, partnership opportunities, and interactive education to o logy and pathology communities. We offer the complete spectrum of diagnostic services in molecular testing, FISH, togenetics, flow cytometry, immunohistochemistry, and morphologic analysis through our world-wide network of CAP-accredited, CLIA-certified laboratories. Committed to research as the means to improve patient care, we provide Pharma Services for pharmaceutical companies, in vitro diagnostic manufacturers, and academic scientist-clinicians. We promote joint publications with our client physicians. NeoGenomics welcomes your inquiries for collaborations. Please contact us for more information. 9490 NeoGenomics Way Fort Myers, FL 33912 Phone: 866.776.5907 www.neogenomics.com © 2022 NeoGenomics Laboratories, Inc. All rights reserved. All trademarks are the property of their respective owners. Rev. 040622 Dze gy r the nco cy