10-K: Annual report pursuant to Section 13 and 15(d)
Published on February 20, 2024
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the fiscal year ended December 31 , 2023
or
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the transition period from _________ to __________
Commission File Number: 001-35756
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) |
(IRS Employer Identification No.) |
|||||||
(Address of principal executive offices, Zip code)
(Registrant’s telephone number, including area code)
| Securities registered pursuant to Section 12(b) of the Act: | Trading Symbol(s): | Name of each exchange on which registered: | ||||||||||||
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
☒ |
Accelerated filer | ☐ | ||||||||||||
| Non-accelerated filer | ☐ | Smaller Reporting Company | ||||||||||||
| Emerging Growth Company | ||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act): ☐ Yes ☒ No
As of June 30, 2023, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was $1.4 billion, based on the closing price of the registrant’s common stock of $16.07 per share on June 30, 2023.
The number of shares outstanding of the registrant’s common stock, par value $0.001 per share, as of February 13, 2024: 127,610,039 .
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for its 2024 Annual Meeting of Stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
NEOGENOMICS, INC.
FORM 10-K ANNUAL REPORT
For the Fiscal Year Ended December 31, 2023
Table of Contents
| Page | ||||||||||||||
2
| NEOGENOMICS, INC. | ||||||||
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements. These forward-looking statements generally can be identified by the use of words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intends,” “may,” “plan,” “potential,” “project,” “will,” “would,” and similar expressions, although not all forward-looking statements contain these identifying words. These forward-looking statements address various matters, including the Company’s strategy, future operations, future financial position, future revenues, changing reimbursement levels from government payers and private insurers, projected costs, prospects and plans, and objectives of management. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. These forward-looking statements involve known and unknown risks and uncertainties that could cause our actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation, the risks set forth below under “Risk Factors Summary” and “Risk Factors” in Part I, Item 1A in this Annual Report on Form 10-K as filed with the Securities and Exchange Commission (the “SEC”).
The forward-looking statements included in this Annual Report on Form 10-K speak only as of the date of this report, and the Company undertakes no obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time and it is not possible for management to predict all of such factors, nor can it assess the impact of each such factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements.
Trademarks
The “NeoGenomics”, “Genoptix”, “Clarient”, “Inivata”, and “Trapelo” company names and certain logos have been trademarked with the United States Patent and Trademark Office. We have trademarked or have applications pending for the brand names NEO COMPREHENSIVE, NEO EXPAND, NEOLINK, NEOLAB, NEOACCESS, NEOTYPE, NEOSITE, CHART, COMPASS, FLEXREPORT, HEMEFISH, MULTIOMYX, NEOVUE, NEOLYTX, NEOACCELERATE, NEOENGAGE, NEOPIXEL, NEONUCLEUS, NEOSEEK, NEOEXPLORE, NEOUNIVERSITY, PATHSITE, QUICKPATH, TAM-SEQ, ETAM-SEQ, INVISION, INVISIONSEQ, INVISIONFIRST, INVISIONSCAN, RADAR, and NEORADAR. We also have trademarked or have pending trademarks for the marketing slogans “SERVING PATIENTS, SAVING LIVES”, “PUTTING THE CAN IN CANCER”, “TAKING CANCER PERSONALLY”, and “UNIVERSAL FUSION EXPRESSION”. Any other trademarks, registered marks and trade names appearing in this Annual Report on Form 10-K are the property of their respective holders.
3
| NEOGENOMICS, INC. | ||||||||
Glossary
Throughout this 2023 Form 10-K, we may use certain abbreviations, acronyms and terms which are described below:
| ACA | The Patient Protection and Affordable Care Act | |||||||
| ACLA | American Clinical Laboratory Association | |||||||
| AKS | Anti-Kickback Statute | |||||||
| CAP | College of American Pathologists | |||||||
| CDx | Companion Diagnostic | |||||||
| CLIA | Clinical Laboratory Improvement Amendments of 1988 | |||||||
| CMS | Centers for Medicare and Medicaid Services | |||||||
| CRO | Contract research organizations | |||||||
| DHS | Designated health services | |||||||
| FCA | The federal False Claims Act | |||||||
| FDA | U.S. Federal Drug Administration | |||||||
| FISH | Fluorescence In-Situ Hybridization | |||||||
| GAAP | U.S generally accepted accounting principles | |||||||
| GDPR | The European Union’s General Data Protection Regulation | |||||||
| HIPAA | The Health Insurance Portability and Accountability Act of 1996 | |||||||
| IHC | Immunohistochemistry | |||||||
| LDT | Laboratory developed tests | |||||||
| LIMS | Laboratory Information Management System | |||||||
| MolDx | Molecular Diagnostic Services Program | |||||||
| MRD | Minimal residual disease | |||||||
| NGS | Next-generation sequencing | |||||||
| OIG | The Office of Inspector General of the Department of Health and Human Services | |||||||
| PCR | Polymerase chain reaction | |||||||
| PHI | Protected health information | |||||||
4
| NEOGENOMICS, INC. | ||||||||
PART I
ITEM 1. BUSINESS
NeoGenomics, Inc., a Nevada corporation (referred to individually as the “Parent Company” or collectively with its subsidiaries as “NeoGenomics”, “we”, “us”, “our”, or the “Company” in this Annual Report on Form 10-K) is the registrant for SEC reporting purposes. Our common stock is listed on The Nasdaq Stock Market LLC (“Nasdaq”) under the symbol “NEO”.
Overview
NeoGenomics provides a wide range of oncology diagnostic testing and consultative services which includes technical laboratory services and professional interpretation of laboratory test results by licensed physicians who specialize in pathology and oncology. We operate a network of cancer-focused testing laboratories in the United States and the United Kingdom. Our mission is to save lives by improving patient care. Our vision is to become the world’s leader in cancer testing, information, and decision support by providing uncompromising quality, exceptional service, and innovative solutions.
As of December 31, 2023, the Company operated College of American Pathologists (“CAP”) accredited and Clinical Laboratory Improvement Amendments of 1988 (“CLIA”) certified laboratories in Fort Myers, Florida; Aliso Viejo and San Diego, California; Research Triangle Park, North Carolina; and Houston, Texas; and a CAP accredited full-service, sample-processing laboratory in Cambridge, United Kingdom. We also have several, small, non-processing laboratory locations across the United States for providing analysis services. We currently offer the following types of testing services:
•Cytogenetics (“karyotype analysis”) – the study of normal and abnormal chromosomes and their relationship to disease. Cytogenetics involves analyzing the chromosome structure to identify changes from patterns seen in normal chromosomes. Cytogenetic studies are often performed to provide diagnostic, prognostic and occasionally predictive information for patients with hematological malignancies.
•Fluorescence In-Situ Hybridization (“FISH”) – a molecular cytogenetic technique that focuses on detecting and localizing the presence or absence of specific DNA sequences and genes on chromosomes. The technique uses fluorescent probes that bind to only those parts of the chromosome with which they show a high degree of sequence similarity. Fluorescence microscopy is used to visualize the fluorescent probes bound to the chromosomes. FISH can be used to help identify numerous types of gene alterations, including amplifications, deletions, and translocations.
•Flow cytometry – a technique utilized to measure the characteristics of cell populations. Typically performed on liquid samples such as peripheral blood and bone marrow aspirate, it may also be performed on solid tissue samples such as lymph nodes following additional processing steps. Cells are labeled with selective fluorescent antibodies and analyzed as they flow in a fluid stream through a beam of light. The properties measured in these antibodies include the relative size, relative granularity or internal complexity, and relative fluorescence intensity. These fluorescent antibodies bind to specific cellular antigens and are used to identify abnormal and/or malignant cell populations. Flow cytometry is typically utilized in diagnosing a wide variety of hematopoietic and lymphoid neoplasms. Flow cytometry is also used to monitor patients during the course of therapy to identify extremely low levels of residual malignant cells, known as minimal residual disease (“MRD”) monitoring.
•Immunohistochemistry (“IHC”) and Digital Imaging – the process of localizing cellular proteins in tissue sections and relies on the principle of antigen-antibody binding. IHC is widely used in the diagnosis of abnormal cells such as those found in cancer. Specific surface membrane, cytoplasmic, or nuclear markers may be identified. IHC is also widely used to understand the distribution and localization of differentially expressed proteins. Digital imaging allows clients to visualize scanned slides and also perform quantitative analysis for certain stains. Scanned slides are received online in real time and can be previewed often a full day before the glass slides can be shipped back to clients.
•Molecular testing – a rapidly growing field which includes a broad range of laboratory techniques utilized in cancer testing. Most molecular techniques rely on the analysis of DNA and/or RNA, as well as the structure and function of genes at the molecular level. Common molecular testing technologies include: DNA fragment length analysis; polymerase chain reaction (“PCR”) analysis; reverse transcriptase polymerase chain reaction (“RT-PCR”) analysis, real-time (or quantitative) polymerase chain reaction (“qPCR”) analysis; bi-directional Sanger sequencing analysis; and next-generation sequencing (“NGS”) analysis.
•Morphologic analysis – the process of analyzing cells under the microscope by a pathologist, usually for the purpose of diagnosis. Morphologic analysis may be performed on a wide variety of samples, such as peripheral blood, bone marrow, lymph nodes, and other sites such as lung, breast, etc. The services provided at NeoGenomics may include primary diagnosis, in which a sample is received for processing and our pathologists provide the initial diagnosis; or
5
| NEOGENOMICS, INC. | ||||||||
may include secondary consultations, in which slides and/or tissue blocks are received from an outside institution for second opinion. In the latter setting, the expert pathologists at NeoGenomics assist our client pathologists on their most difficult and complex cases.
Reportable Segments
We have analyzed our reporting structure, the information available to our Chief Operating Decision Maker (“CODM”) and the information being used to make strategic decisions and have identified two primary types of customers, served by two distinct company divisions: Clinical Services and Advanced Diagnostics. Our Clinical Services customers include community-based pathology and oncology practices, hospital pathology labs, reference labs, and academic centers. Our Advanced Diagnostics customers include pharmaceutical companies to whom we provide testing and other services to support their research studies and clinical trials.
In 2023, our Clinical Services segment accounted for 84% of consolidated revenue and our Advanced Diagnostics segment accounted for 16% of consolidated revenue. Please refer to Note 17. Segment Information, to our Consolidated Financial Statements included in this Annual Report for further financial information about these segments.
Clinical Services Segment
Our Clinical Services revenue consists of the following four revenue streams:
•Clinical cancer testing;
•Interpretation and consultative services;
•Molecular and NGS testing; and
•Comprehensive technical and professional services offering.
The cancer testing services we offer to community-based pathologists and oncologists are designed to be a natural extension of, and complementary to, the services that they perform within their own practices. We believe our relationship as a non-competitive partner to community-based pathology practices, hospital pathology labs, reference labs, and academic centers can empower them to expand their breadth of testing to provide a menu of services that could match or exceed the level of service found in any center of excellence around the world. Community-based pathology practices and hospital pathology labs may order certain testing services on a technical component only (“TC” or “tech-only”) basis, which allows them to participate in the diagnostic process by performing the professional component (“PC”) interpretation services without having to hire laboratory technologists or purchase the sophisticated equipment needed to perform the technical component of the tests. We also support our pathology clients with interpretation and consultative services using our own specialized team of pathologists for difficult or complex cases and we provide overflow interpretation services when requested by clients.
In addition, we directly serve oncology, dermatology and other clinician practices that prefer to have a direct relationship with a laboratory for cancer-related genetic testing services. We typically serve these types of clients with a comprehensive service offering where we perform both the technical and professional components of the tests ordered. In certain instances, larger clinician practices have begun to internalize pathology interpretation services, and our tech-only service offering allows these larger clinician practices to also participate in the diagnostic process by performing the PC interpretation services on TC testing performed by us.
We believe we are a leading provider of Heme Molecular and NGS testing and one of the key providers of solid tumor NGS testing solutions. These tests are interpreted by NeoGenomics’ team of molecular experts and are often ordered in conjunction with other testing modalities. NGS panels are one of our fastest growing testing areas, and clients can often receive a significant amount of biomarker information from very limited samples. These comprehensive panels can allow for faster treatment decisions for patients as compared to a series of single-gene molecular tests being ordered sequentially. We have a broad molecular testing menu, and our targeted NeoTYPE panels include genes relevant to a particular cancer type. These tests are complemented by IHC and FISH tests, as necessary. In addition, we offer molecular-only NGS-targeted and comprehensive panels which combine DNA and RNA into a single work stream in order to report a full spectrum of genomic alterations, including mutations, fusions, copy number variations, and splicing mutations, as well as tumor mutation burden (TMB) and microsatellite instability (MSI) for solid tumor cases. This comprehensive molecular test menu allows our clients to obtain most of their molecular oncology testing needs satisfied by our laboratory. This is attractive to our clients as patient samples do not need to be split and then managed across several laboratories. The acquisition of Inivata in June of 2021 provided us with oncology liquid biopsy technology capabilities. InVisionFirst®-Lung is a highly sensitive, targeted plasma-based assay for patients with non-small cell lung cancer, and RaDaR® is a liquid biopsy assay designed to detect residual disease and recurrence in plasma samples from patients with solid tumor malignancies. We expect our molecular laboratory and NGS capabilities to be a key growth driver in the coming years.
6
| NEOGENOMICS, INC. | ||||||||
Advanced Diagnostics Segment
In the second quarter of 2023, the Advanced Diagnostics segment was rebranded as the Advanced Diagnostics segment.
Our Advanced Diagnostics revenue consists of the following three revenue streams:
•Clinical trials and research;
•Validation laboratory services; and
•Informatics.
Our Advanced Diagnostics segment supports pharmaceutical firms in their drug development programs by supporting various clinical trials and research. This portion of our business often involves working with the pharmaceutical firms (“sponsors”) on study design as well as performing the required testing. Our medical team often advises the sponsor and works closely with them as specimens are received from the enrolled sites. We also work on developing tests that will be used as part of a companion diagnostic to determine patients’ responses to a particular drug. As studies unfold, our clinical trials team reports the data and often provides key analysis and insights back to the sponsors.
Our Advanced Diagnostics segment provides comprehensive testing services in support of our pharmaceutical clients’ oncology programs from discovery to commercialization. In biomarker discovery, our aim is to help our customers discover the right content. We help our customers develop a biomarker hypothesis by recommending an optimal platform for molecular screening and backing our discovery tools with the informatics to capture meaningful data. In other pre-clinical and non-clinical work, we can use our platforms to characterize markers of interest. Moving from discovery to development, we seek to help our customers refine their biomarker strategy and, if applicable, develop a companion diagnostic pathway using the optimal technology for large-scale clinical trial testing.
Whether serving as the single contract research organization or partnering with one, our Advanced Diagnostics team provides significant technical expertise, working closely with our customers to support each stage of clinical trial development. Each trial we support comes with rapid turnaround time, dedicated project management and quality assurance oversight. We have experience in supporting submissions to the Federal Drug Administration (“FDA”) for companion diagnostics. Our Advanced Diagnostics strategy is focused on helping to bring more effective oncology treatments to market through providing world-class laboratory services in oncology to key pharmaceutical companies in the industry.
We believe that we are well positioned to service sponsors across the full continuum of the drug development process. Our Advanced Diagnostics team can work with these sponsors during the basic research and development phase as compounds come out of translational research departments, as well as work with clients from Phase I, Phase II and Phase III clinical trials as the sponsors work to demonstrate the efficacy of their drugs. The laboratory biomarker tests that are developed during this process may become companion diagnostic (“CDx”) tests that will be used on patients to determine if they could respond to a certain therapy. We are able to offer these CDx tests to the market immediately after FDA approval as part of our Day 1 readiness program. This ability helps to speed the commercialization of a drug and can enable sponsors to reach patients through our broad distribution channel in the Clinical Services segment.
We are committed to connecting patients with life-altering therapies and trials. In carrying out these commitments, we aim to provide transparency and choice to patients regarding the handling and use of their data through our Notice of Privacy Practices, and have invested in leading technologies to secure the data we maintain. We are continuing to develop and broaden our informatics and data-related tools to leverage our unique market position and oncology expertise to help our stakeholders solve real-world problems such as identifying patients for clinical trials or providing clinical decision support tools for physicians and providers.
Markets
The medical testing laboratory market can be broken down into the following three primary markets:
•Clinical Pathology testing;
•Anatomic Pathology testing; and
•Genetic and Molecular testing.
Clinical Pathology testing covers high volume, automated, lower complexity tests on easily procured specimens such as blood and urine.
Anatomic Pathology testing involves evaluation of tissue, as in surgical pathology, or cells as in cytopathology. The most widely performed Anatomic Pathology procedures include the preparation and interpretation of pap smears, skin biopsies, and tissue biopsies.
7
| NEOGENOMICS, INC. | ||||||||
Genetic and molecular testing typically involves analyzing chromosomes, genes, proteins, and/or DNA/RNA sequences for abnormalities. Genetic and molecular testing requires highly specialized equipment and credentialed individuals (typically MD or PhD level) to certify results and typically yields the highest reimbursement levels of the three market segments.
NeoGenomics operates primarily in the Genetic and Molecular testing market and the Anatomic Pathology market.
The field of cancer genetics is evolving rapidly and new tests continue to be developed at an accelerated pace. Based on medical and scientific discoveries over the last decade, cancer testing falls into one of three categories: diagnostic testing, prognostic testing and predictive testing. Of the three, the fastest growing area is predictive testing, which is utilized by clinicians to predict which treatment options a patient will be most likely to benefit from in order to deliver “personalized” or “precision medicine” that is optimized to that patient’s particular circumstances. Personalized or precision medicine better allows clinicians to know if a patient will or will not respond to certain cancer medications such as Herceptin®, Keytruda®, PIQRAY®, and Opdivo®, among many others. In addition to the direct benefits to patients, the “precision medicine” approach allows the healthcare system to save money by ensuring that expensive cancer drugs are only given to those who will be most likely to benefit from them. This type of testing improves patient care and potentially saves lives by identifying optimized therapies much more rapidly than what was possible in previous years.
The U.S. market for genetic and molecular testing is divided among numerous laboratories. Many of these laboratories are attached to academic institutions and primarily provide clinical services to their affiliated university hospitals and associated physicians.
We believe several key factors are influencing the rapid growth in the market for cancer testing: (i) every year, more and more genes and genomic pathways are implicated in the development and/or clinical course of cancer; (ii) the incidence rates of cancer are rising, notably in younger populations, and more than one in three citizens is likely to develop some form of cancer in their lifetime; (iii) increasingly, new drugs are being targeted to certain cancer subtypes and pathways which require companion diagnostic testing; (iv) patient and payer awareness of the value of genetic and molecular testing; (v) decreases in the cost of performing genetic and molecular testing; (vi) increased coverage from third party payers and Medicare for such testing; and (vii) the health insurance coverage to uninsured Americans under the Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act, each enacted in March 2010. These factors have driven significant growth in the market for this type of testing. Additionally, there is an increased focus on developing tests for monitoring purposes, including MRD and recurrence detection in cancer survivors, which could also broaden the use of certain tests and influence the market for cancer testing.
Intellectual Property
Protection of our intellectual property is fundamental to the long-term success of our business. We rely on a combination of patents, trademarks, copyrights, trade secrets, license agreements, confidentiality agreements and procedures, non-disclosure agreements, and other contractual rights and obligations to protect the investments made into the development of our technology.
Issued U.S. patents and their international counterparts currently in our patent portfolio that relate to various aspects of our technology and products are expected to expire between 2025 and 2036.
To protect our brand and identity, the “NeoGenomics”, “Genoptix”, “Clarient”, “Inivata”, and “Trapelo” company names and certain logos have been trademarked with the United States Patent and Trademark Office.
We intend to pursue additional intellectual property protection to the extent we believe it would advance our business objectives. Despite our efforts to protect our intellectual property rights, however, we may not be successful and our intellectual property rights may be invalidated, circumvented or challenged and found unenforceable.
We also rely on trade secrets, including know-how, to protect our unpatented technology and other proprietary information, and to maintain and strengthen our competitive position. To mitigate the risk of trade secret misappropriation, it is our policy to enter into nondisclosure and confidentiality agreements with parties who have access to our trade secrets, such as our employees, collaborators, outside scientific collaborators, consultants, advisors and other third parties. We also enter into invention disclosure and assignment agreements with our employees and consultants that obligate them to assign to us any inventions they have developed while working for us.
2024 Focus Areas:
We are committed to sustainable growth while transforming cancer care for patients and providers. Our focus for 2024 is to sustain a purpose driven culture that maintains excellence in service and performance while growing through innovation. We expect the following initiatives to allow the Company to continue on its path to become one of the world’s leading cancer testing and information companies:
8
| NEOGENOMICS, INC. | ||||||||
Profitably Grow Core Business
•Grow volume and NGS mix;
•Drive market penetration;
•Win on oncology; and
•Improve revenue cycle management.
Accelerate Advanced Diagnostics
•Execute Neo Comprehensive 2.0 launch;
•Execute liquid biopsy Comprehensive Genetic Profiling (“CGP”) launch; and
•Improve gross margin.
Drive Value Creation
•Increase productivity and efficiency;
•Improve gross margin;
•Implement Laboratory Information Management System (“LIMS”) Phase 1; and
•Prioritize quality system enhancements.
Enhance Our People and Culture
•Enhance teammate development and engagement; and
•Grow a customer-oriented and growth mindset.
Competitive Strengths
In addition to the competitive strengths discussed below, we believe that our superior testing technologies and instrumentation, laboratory information system, client education programs and domestic and international presence also differentiate NeoGenomics from its competitors.
Turnaround Times
We consistently focus on improving turnaround times for test results to our clients nationwide in the Clinical Services segment. By providing information to our clients in a timely manner, physicians can begin treating their patients as soon as possible. Timeliness of results by our Clinical Services segment is a driver of additional testing requests by referring physicians. Turnaround times allow for the performance of other adjunctive tests within an acceptable diagnosis window in order to augment or confirm results and more fully inform treatment options. Additionally, we believe that our rapid turnaround time on testing and our project milestones are a key factor in our Advanced Diagnostics segment.
Innovative Service Offerings
We believe we currently have one of the most extensive menu of tech-only FISH services in the United States as well as extensive and advanced tech-only flow cytometry and IHC testing services. These types of testing services allow the professional interpretation component of a test to be performed and billed separately by our physician clients. Our tech-only services are designed to give pathologists the option to choose, on a case by case basis, whether they want to order only the technical component of testing so they can perform the professional interpretation, or order “global” services and receive a comprehensive test report which includes a NeoGenomics pathologist’s interpretation of the test results. Our clients appreciate the flexibility to access NeoGenomics’ medical staff for difficult or complex cases or when they are otherwise unavailable to perform professional interpretations.
We offer a comprehensive suite of technical and professional interpretation services to meet the needs of clients who are not credentialed and/or trained in interpreting various testing modalities and who require NeoGenomics' pathology specialists to interpret their testing results. In our global service offerings, our lab performs the technical component of testing and our MDs and PhDs provide the professional component of testing by interpreting the results of those tests. Our professional staff is also available for post-test consultative services. Clients using our global service offering rely on the expertise of our medical team to give them the answers they need in a timely manner to help inform their diagnoses and treatment decisions.
Our Molecular and NGS Clinical Services segment test menus provide clients with the ability to order single gene molecular tests, targeted NeoTYPE panels that include the relevant actionable genes for a particular cancer type, as well as comprehensive NGS panels. Our Advanced Diagnostics segment offers a full range of sequencing testing including whole exome and whole genome sequencing.
National Direct Sales Force
9
| NEOGENOMICS, INC. | ||||||||
Our direct sales force has been trained extensively in cancer genetic testing and consultative selling skills to service the needs of clients. Our sales team for the Clinical Services segment is organized into nine regions in the United States – Northeast, Northwest, Mid-Atlantic, South, Southeast, North Central, West, Great Lakes, and South Central. Our sales team will be focused on value-based care solutions and end-to-end client experience as a growth driver. Our Advanced Diagnostics segment has a dedicated team of business development specialists who are experienced in working with sponsors and helping them with the testing needs of their research and development projects as well as Phase I, II and III studies. These sales representatives utilize our custom Customer Relationship Management System (“CRM”) to manage their territories, and we have integrated the key customer care functionality within our LIMS into the CRM so that our sales representatives can stay informed of emerging issues and opportunities within their regions. Our in-house customer care team is aligned with our field sales team to serve the needs of our clients by utilizing the same LIMS and CRM. Our field teams can see in real time when a client calls the laboratory, the reason for the call and the resolution, and determine if face-to-face interaction is needed for follow-up. Our sales force educates clients on new test offerings and their proper utilization and our representatives are often seen as trusted advisors by our clients.
Seasonality
The majority of our clinical testing volume is dependent on patients being treated by hematology/oncology professionals and other healthcare providers. The volume of our testing services generally declines modestly during the summer vacation season, year-end holiday periods and other major holidays, particularly when those holidays fall during the middle of the week. In addition, the volume of our testing tends to decline due to extreme adverse weather conditions, such as excessively hot or cold spells, heavy snow, hurricanes or tornadoes in certain regions, consequently reducing revenues and cash flows in any affected period.
In our Advanced Diagnostics segment, we enter into both short-term and long-term contracts, ranging from one month to several years. While the volume of this testing is not as directly affected by seasonality as described above, the testing volume does vary based on the terms of the contract. Our volumes are often based on how quickly sponsors can get patient enrollees for their trials and seasonality can impact how quickly patients are enrolled. Many of our long-term contracts contain specific performance obligations where the testing is performed on a specific schedule. In addition, this results in backlog that can be significant and highly dependent on pharmaceutical clinical trial enrollment.
Competition
Our competitors within the broader genomics profiling space include laboratory companies such as Quest Diagnostics, Laboratory Corporation of America and Bio-Reference Laboratories. These are large national laboratories that possess greater name recognition, larger customer bases, and significantly greater financial resources and employ substantially more personnel than we do. We also face increased competition from laboratories that are more specialized and focused on particular areas such as liquid biopsies or large tissue based molecular panels such as Guardant Health, Inc., Natera, Inc., Exact Sciences Corp, Caris Life Science, Tempus Labs, Inc. and Myriad Genetics, Inc.
For our Clinical Services segment, the genetic and molecular testing niche of the laboratory testing industry is highly competitive and, given the opportunities in this industry, we expect it to become even more competitive. Competitive factors in genetic and molecular testing generally include the reputation of the laboratory, range of services offered, pricing, convenience of sample collection and pick-up, quality of analysis and reporting, medical staff, timeliness of delivery of completed reports (i.e. turnaround times) and post-reporting follow-up for clients.
Our competitors for our Clinical Services segment in the United States are numerous and include major national medical testing laboratories, hospital laboratories and in-house physician laboratories. Some of our competitors have greater financial resources and production capabilities than us. These companies may succeed in developing service offerings that are more effective than any that we have or may develop, and may also prove to be more successful than we are in marketing such services. In addition, technological advances or different approaches developed by one or more of our competitors may render our service offerings obsolete, less effective or uneconomical.
We intend to continue our efforts to gain market share by offering a broad portfolio of tests with rapid turnaround times, wrap-around services, and solutions targeted to hospitals and community oncology segments. The addition of new tests (including proprietary ones), enhanced post-test consultation services, and personal attention from our direct sales force will further enhance our efforts.
Our Advanced Diagnostics business competes against many other Contract Research Organizations (“CROs”) and central reference laboratories. Many of these competitors are much larger and have a greater international presence than we do. Over the past few years, we have expanded our Advanced Diagnostics business into Europe at the request of our clients and we
10
| NEOGENOMICS, INC. | ||||||||
believe that our expansive oncology testing menu and our high level of service will allow us to continue to gain market share in this segment.
Many clinical reference laboratories have also entered the space in support of clinical trials and the related laboratory testing. These reference laboratories are often willing to compete with lower pricing for smaller, more limited studies. We believe our strong scientific and medical team is a key differentiator where NeoGenomics is used as an advisor to the sponsors on their trials. Our extensive experience in anatomic pathology continues to result in our winning clinical trials business as sponsors trust our medical team and want them to closely oversee their trials. We believe our service focus and our molecular and IHC platforms, as well as our exclusive MultiOmyxTM platform will continue to lead to rapid growth in this segment.
Suppliers
We order our laboratory and research supplies from large national laboratory supply companies. While we do not depend on a concentrated, limited number of suppliers, we do rely on certain suppliers for specific reagents or other equipment, including sequencers. While we do not believe a short-term disruption from any one of these suppliers would have a material effect on our business, it could result in short-term impact on our turnaround time or gross margin depending on the nature or extent of the disruption.
Concentrations of Credit Risk
Concentrations of credit risk with respect to revenue and accounts receivable are primarily limited to certain clients to which we provide a significant volume of our services, and to specific payers of our services such as Medicare and individual insurance companies.
Dependence on Major Clients
We market our services to pathologists, oncologists, other clinicians, hospitals, pharmaceutical companies, academic centers and other clinical laboratories throughout the United States and the United Kingdom. Our client base consists of a large number of geographically dispersed clients diversified across various customer types. For the years ended December 31, 2023, 2022 and 2021, no single client accounted for more than 10% of revenue.
Payer Mix
The following table reflects our estimate of the breakdown of net clinical revenue by type of payer for the years ended December 31, 2023, 2022 and 2021:
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Client direct billing | 67 | % | 67 | % | 63 | % | ||||||||||||||
| Commercial insurance | 18 | % | 17 | % | 19 | % | ||||||||||||||
| Medicare and other government | 15 | % | 16 | % | 18 | % | ||||||||||||||
| Total | 100 | % | 100 | % | 100 | % | ||||||||||||||
All of our Advanced Diagnostics revenue is billed directly to clients or the pharmaceutical sponsor.
Insurance
We maintain professional liability and numerous other insurance policies. We believe that our present insurance is sufficient to cover currently estimated exposures, but we cannot assure that we will not incur liabilities in excess of the policy coverage limits. In addition, although we believe that we will be able to continue to obtain adequate insurance coverage, we cannot assure that we will be able to do so at an acceptable cost.
Human Capital Management
As of December 31, 2023, we had approximately 2,100 full-time equivalent employees and contracted pathologists.
World-Class Medical and Scientific Team
Individuals comprising our medical and scientific team are specialists in the field of genetics, oncology and pathology. As of December 31, 2023, we employed or contracted with approximately 170 MDs and PhDs. We have many nationally and world-renowned pathologists on staff, which is a key differentiator from many smaller laboratories. Our clinical customers look to our staff and their expertise, and they often call our medical team on challenging cases. For our Advanced Diagnostics segment, many sponsors work with our medical team on their study design and on the interpretation of results from the
11
| NEOGENOMICS, INC. | ||||||||
studies. Our medical team is a key differentiator as we have a depth of medical expertise that many other laboratories cannot offer to pharmaceutical companies.
World-Class Culture
We promote a world-class culture through employee engagement, training and development, wellness, work-life balance, and communication initiatives. Human capital management, including the recruitment and retention of a talented, diverse and highly motivated workforce, is an essential component of our strategy for long-term value creation. We value our teammates and focus on driving employee engagement through internal programs, external outreach, and other internal collaborative initiatives.
Our commitment to maintaining an excellent workplace includes investing in ongoing opportunities for employee development in a diverse and inclusive environment. In addition to gender and ethnic diversity and inclusion on our Board of Directors, diversity in gender and ethnicity is well-established within our workforce. As of December 31, 2023, women make up 59.9% of our global workforce, and of the 17.8% of our workforce that is in supervisory or higher positions, 50.8% are female. With regard to the Company’s top two management tiers, 44.4% of our executive team and our vice presidents are women and 33.3% of our independent Board of Directors are women. Ethnicity is also strongly represented: 44.6% of our workforce and 25.0% of our independent Board of Directors are ethnically diverse.
We believe that a diverse and inclusive workforce, where all perspectives are recognized and respected, positively impacts our performance and strengthens our culture. We continuingly strive to promote a workplace in which people of diverse race, ethnicity, veteran status, marital status, socioeconomic level, national origin, religious belief, physical ability, sexual orientation, age, class, political ideology, gender identity and expression participate in, contribute to, and benefit equally.
We are also committed to rewarding, supporting and developing our employees as they work toward our common purposes of saving lives by improving patient care. To that end, we offer a competitive comprehensive rewards package that includes competitive salaries, performance-based bonuses, equity grants, healthcare benefits, retirement savings plans, paid family leave, paid time off, wellness programs and discounts, tuition reimbursement and an Employee Assistance Program. We also drive high levels of performance and improvement by prioritizing training and development, and we motivate and develop our employees by providing them with opportunities for advancement and offering robust onsite and remote learning opportunities for employees at every stage in their career.
Government Regulation
The laboratory industry is subject to extensive governmental regulation domestically, at the federal and state levels, and internationally. The applicable laws and regulations change frequently and there can be no assurance that the Company will not be subject to audit, inquiry, or investigation with respect to some aspect of its operations. The failure to comply with applicable laws, regulations, and reimbursement guidelines could have a material adverse effect on the Company’s business. Significant areas of regulation are summarized below. For additional information about government regulation and laws applicable to our business, see “Item 1A. Risk Factors,” including the risk factors entitled “Risks Relating to Government Regulation and Reimbursement.”
Licensure, Accreditation, and Quality Standards
The Company operates laboratories domestically in Arizona, California, Florida, Georgia, Illinois, North Carolina, Tennessee, and Texas, and internationally in the United Kingdom. The laboratories are licensed as required by the states or countries in which they are located. In addition, the laboratories in Fort Myers, Florida; Aliso Viejo and Carlsbad, California; Nashville, Tennessee; and Houston, Texas are licensed by the State of New York as the laboratories accept clinical specimens obtained in New York. All of our domestic laboratories are certified in accordance with the CLIA. Under CLIA, the Centers for Medicare & Medicaid Services (“CMS”) establish various operational, personnel, facilities, administration, quality, and proficiency requirements for testing performed by a laboratory, intended to ensure testing services are accurate, valid, and timely. CLIA certification is also a prerequisite to be eligible to bill federal and state health care programs, as well as many private insurers, for laboratory testing services. The sanctions for failure to comply with CLIA requirements include: suspension, revocation, or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business; cancellation or suspension of the laboratory’s approval to receive Medicare and/or Medicaid reimbursement; and significant fines and/or criminal penalties. The loss or suspension of a CLIA certification could have a material adverse effect on the Company.
Our laboratory in Cambridge, United Kingdom is accredited by CAP and actively participates in CAP’s proficiency testing programs for all tests offered by the Company. CAP’s proficiency testing programs require participating laboratories to test specimens that they receive from an approved testing entity and return the results. The testing entity conducting the program analyzes the results and provides to the Company a quality control report assessing the results.
12
| NEOGENOMICS, INC. | ||||||||
The Company has a Quality Management System, and we strive to conduct our business in a manner that meets applicable regulatory and accreditation requirements and industry standards. The quality of care provided to clients and their patients is of paramount importance to us. We maintain quality control processes, including standard operating procedures, controls, performance measurement and reporting mechanisms. Our employees are committed to providing accurate, reliable, and consistent services. Any concerns regarding the quality of testing or services provided by the Company are quickly communicated to our Company management. We also frequently revise and improve our tests, and we work with laboratory equipment vendors to help ensure that our laboratory has the highest possible quality.
Compliance with licensure, accreditation, and quality standards are verified through periodic inspections by agents of relevant regulatory agencies and accrediting organizations, and we believe we are in material compliance with all licensure, accreditation, and quality requirements.
Compliance and Ethics Program
The health care industry is highly regulated and scrutinized with respect to fraud, abusive billing practices, and improper financial relationships between health care companies and their referral sources. The Office of Inspector General of the Department of Health and Human Services (“OIG”) has published compliance program guidance, including a General Compliance Program Guidance issued in November 2023 and applicable to all health care companies and stakeholders, a specific Compliance Program Guidance for Clinical Laboratories issued in August of 1998 (which the OIG plans to update in the coming years), fraud alerts, and advisory opinions. The Company has implemented a robust Compliance & Ethics Program encompassing this guidance, which is overseen by our Board of Directors, to support compliance with the myriad of international, federal, and state laws, regulations, and governmental guidance applicable to our business. Our program employs a risk-based approach to the development and implementation of standards of conduct, training and education of employees, monitoring and auditing Company practices, investigation, and response to reported or detected compliance issues. The Company provides a hotline for employees who wish to anonymously or confidentially report suspected violations of our codes of conduct, policies and procedures, or laws and regulations. Employees are strongly encouraged to report suspected violations. The hotline does not replace other resources available to our employees, including supervisors, managers, and human resources staff but is an alternative channel available 24 hours a day, 365 days a year. The hotline forwards all reports to the Chief Compliance Officer, who is responsible for investigating, reporting to the Compliance Committee, and documenting the disposition of each report. The hotline forwards any calls pertaining to the financial statements or financial issues to the Chairman of the Audit Committee. The Company does not allow any retaliation against an employee who reports a compliance related issue in good faith.
The Board of Directors has a Compliance Committee that meets regularly to discuss all compliance-related issues that may affect the Company. The Company reviews its policies and procedures as new regulations and interpretations come to light to comply with applicable regulations. The Chief Compliance Officer reports quarterly to the Compliance Committee on the effectiveness of the program.
Laboratory Developed Tests
The FDA has regulatory responsibility over instruments, test kits, reagents, and other medical devices used by clinical laboratories to perform diagnostic testing. High complexity and CLIA-certified laboratories such as ours frequently develop testing procedures intended exclusively for use by the developing laboratory to provide diagnostic results to customers. These tests are referred to as laboratory developed tests (“LDTs”). The regulatory framework governing LDTs is evolving, complex, and has been the subject of ongoing debate. LDTs are subject to CMS oversight through its enforcement of CLIA. The FDA has also claimed regulatory authority over LDTs but has historically exercised enforcement discretion with regard to most LDTs offered by CLIA-certified laboratories performing high complexity tests, and has not subjected these tests to FDA rules and regulations governing medical devices, including premarket review requirements.
On September 29, 2023, the FDA published a proposed rule on LDTs in which the FDA proposes to end its historical policy of enforcement discretion for virtually all LDTs in five stages over a four-year period from the date FDA publishes a final rule. In Phase 1 (effective one-year post finalization), laboratories would be required to comply with medical device (adverse event) reporting and correction and removal reporting requirements. In Phase 2, (effective two years post-finalization), laboratories would be required to comply with all other device requirements (including registration and listing, labeling and investigational use exemptions), except for quality system and premarket review requirements. In Phase 3 (effective three years post-finalization), laboratories would be required to comply with quality system requirements (good manufacturing practices). In Phase 4 (effective three and a half years post-finalization, but not before October 1, 2027), laboratories would be required to comply with premarket review requirements for high-risk tests (i.e., tests subject to premarket approval requirements). Finally, in Phase 5 (effective four years post-finalization, but not before April 1, 2028), laboratories would be required to comply with premarket review requirements for moderate- and low-risk tests (i.e., tests subject to de novo or full
13
| NEOGENOMICS, INC. | ||||||||
510(k) prenotification requirements). Unlike previous FDA proposals, the proposed rule does not “grandfather” any currently marketed tests. The content and timing of any final rule on LDTs is uncertain at this time.
Congress has also considered a number of legislative proposals in recent years that would amend the regulatory framework for LDTs, including, among other requirements, FDA premarket review of certain LDTs. The most recent such proposal, the VALID Act, was introduced in both the House and Senate on June 24, 2021. The VALID Act was expected to be included in the Omnibus bill signed at the end of 2022, but ultimately was not included. The VALID Act was then reintroduced in March 2023. The bill would subject many LDTs to FDA regulation by creating a new in vitro clinical test, or IVCT, category of regulated products. As proposed, the bill would grandfather many existing LDTs from the proposed premarket approval, quality system, and labeling requirements, respectively, but would require such tests to comply with other regulatory requirements (including registration and listing, adverse event reporting). To market a high-risk IVCT, reasonable assurance of analytical and clinical validity for the intended use would be needed to be established. Under the VALID Act, a precertification process would be established that would allow a laboratory to establish that the facilities, methods, and controls used in the development of its IVCTs meet quality system requirements. If pre-certified, low-risk IVCTs, developed by the laboratory would not be subject to pre-market review. The new regulatory framework would include quality control and post-market reporting requirements. The FDA would have the authority to withdraw approvals for IVCTs for various reasons, including (for example) if there were a reasonable likelihood that the test would cause death or serious adverse health consequences. We cannot predict if the VALID Act (or any other bill) will be enacted in its current (or any other) form.
It is possible that changes to FDA’s regulatory approach, whether triggered by legislation or not, may result in increased regulatory burdens and costs for us to seek marketing authorization for and maintain ongoing compliance for our existing tests, any modifications thereto, or any future tests we may develop. If the government begins to regulate our tests, it could require a significant volume of applications, which would be burdensome and potentially costly. Furthermore, governmental bodies could take a long time to review such applications and/or document responses if other laboratories were also required to file applications and/or document responses for each of their LDTs. In addition, we could be required to conduct additional clinical trials in order to support required applications, which could add cost, delay and uncertainty to the process of bringing our tests to market and maintaining compliance of our marketed tests.
We cannot be certain as to which of our tests, if any, would require FDA approval or clearance under any of the proposed frameworks and, if required, that our tests could obtain such approval or clearance.
Laws Governing Source Relationships
The federal laws governing Medicare, Medicaid, and other federal health benefits, as well as other state and federal laws, regulate certain aspects of the relationships between health care providers, including clinical laboratories, and their referral sources, including physicians, hospitals, other laboratories, and other entities. We are subject to the federal Anti-Kickback Statute (“AKS”), which is a criminal felony statute, as well as similar state statutes and regulations, which prohibits the knowing and willful offer, payment, solicitation, or receipt of any form of remuneration in return for referring, ordering, leasing, purchasing, or arranging for or recommending the ordering, purchasing, or leasing of items or services payable by Medicare, Medicaid, or any other federally funded healthcare program. Remuneration has been broadly interpreted to include anything of value, in cash or in kind, and thus can implicate financial relationships including payments not commensurate with fair market value, such as in the form of personnel, supplies, professional, or technical services, or anything else of value. If we are found to be in violation of the AKS or a similar state anti-kickback law, we could be subject to significant penalties, including fines, exclusion from participation in government and private payer programs, or obligations to refund amounts previously received from government payers. For additional information regarding the federal AKS and similar state anti-kickback laws, see Item 1A. Risk Factors, Risks Relating to Government Regulation and Reimbursement, “The failure to comply with Anti-Kickback laws may subject us to liability, penalties or limitation of operations.”
In addition to the federal AKS, in October 2018, the U.S. Congress enacted the Eliminating Kickbacks in Recovery Act of 2018 (“EKRA”), as part of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (“SUPPORT Act”). EKRA is an all-payer anti-kickback law that makes it a criminal offense to pay any remuneration to induce referrals to, or in exchange for, patients using the services of a recovery home, a substance use clinical treatment facility, or laboratory. As drafted, an EKRA prohibition on incentive compensation to sales employees, payments to group purchasing organizations (“GPOs”), or group practices is broader than the federal AKS. Significantly, EKRA permits the U.S. Department of Justice (the “DOJ”) to issue regulations clarifying EKRA’s exceptions or adding additional exceptions, but such regulations have not yet been issued.
We are also subject to international laws and regulations, including the U.S. Foreign Corrupt Practices Act (“FCPA”) and the U.K. Bribery Act, relating to corrupt and illegal payments to, and contracting practices with regard to, government officials
14
| NEOGENOMICS, INC. | ||||||||
and others. The scope of the types of payments or other benefits covered by these laws is very broad and regulators are frequently using enforcement proceedings to define the scope of these laws. These laws include civil penalties for enterprises and criminal penalties and imprisonment for individuals. Any violation of these laws, or allegations of such violations, could disrupt our operations, involve significant management distraction, cause us to incur significant costs and expenses, including legal fees, and result in a material adverse effect on our business. We could also suffer severe penalties, including criminal and civil penalties, disgorgement and other remedial measures. The obligation of the Company under these laws is to screen third parties who are hired to carry out certain services on behalf of the Company, to monitor for and report suspicious transactions, and to monitor direct and indirect payments to government officials and others. Because of the broad definitions of applicability of these laws, international clients or vendors working for government-owned entities are often considered to be governmental officials. The Company has implemented a program to comply with these laws and educates employees and its relevant vendors regularly on the requirements for vendor onboarding and conducting appropriate business interactions globally.
Physician Self-Referral Laws
The federal law referred to as the “Stark Law” prohibits payments for certain health care services, referred to as designated health services (“DHS”), rendered by entities with which referring physicians (or their immediate family members) have a financial relationship. A “financial relationship” includes both an ownership interest in and/or a compensation arrangement with a physician, both direct and indirect, and DHS includes, but is not limited to, laboratory services.
The Stark Law prohibits an entity that receives a prohibited DHS referral from seeking payment from Medicare and Medicaid for any DHS services performed as a result of such a referral, unless an arrangement is carefully structured to satisfy every requirement of a regulatory exception. The Company endeavors to structure its financial relationships in compliance with the Stark Law and with similar state physician self-referral laws, and performs routine audits in furtherance of this compliance.
Many states have promulgated self-referral laws and regulations similar to the federal Stark Law, but these vary significantly based on the state. In addition to services reimbursed by Medicaid or government payers, these state laws and regulations can encompass services reimbursed by private payers and paid by self-pay patients as well. Penalties for violating state self-referral laws and regulations vary based on the state but often include civil and criminal penalties, exclusion from Medicaid, and loss of licenses. Our financial arrangements with physicians are governed by the federal Stark Law and similar state self-referral laws, and we rely on certain exceptions to the Stark Law with respect to such relationships. If we are found to be in violation of the Stark Law or a similar state self-referral law, we could be subject to significant penalties, including fines, exclusion from participation in government and private payer programs, or obligations to refund amounts previously received from government payers.
The False Claims Act
The federal False Claims Act (“FCA”) prohibits any person or entity from knowingly presenting, or causing to be presented, to the U.S. government, or to a Medicare program contractor, a false or fraudulent claim for payment, knowingly making or using a false record or statement to have a false claim paid by the government, conspiring to defraud the U.S. government, or knowingly making or using a false statement to conceal an obligation to pay the government, or improperly retaining overpayments from the government. Following enactment of the Patient Protection and Affordable Care Act (“ACA”), claims resulting from violations of the federal AKS and knowing retention of overpayments are also considered false claims and could lead to liability under the FCA. Further, FCA liability may lead to exclusion from participation in Medicare, Medicaid, and other federal healthcare programs. The FCA’s “whistleblower” or “qui tam” provisions are used with frequency to challenge the reimbursement practices of providers and suppliers. These provisions allow a private individual to bring an action on behalf of the government alleging that the defendant has submitted false claims for payment to the federal government. The government must decide whether to intervene in the lawsuit and whether to prosecute the case. If it declines to do so, the individual may pursue the case alone, although the government must be kept apprised of the progress of the lawsuit. Whether or not the federal government intervenes in the case, it will receive the majority of any recovery. The successful qui tam relator who brought the case is entitled to a portion of the proceeds and its attorneys’ fees and costs. As most qui tam cases are filed by current or former employees, an effective compliance program, as defined by the DOJ and OIG, plays a crucial role in reducing the Company’s exposure to liability. It is also a criminal offense, under Title 18 U.S. Code, Section 287, for a person or entity to make a claim against the United States or any department or agency, knowing the claim to be false, fictitious, or fraudulent. The penalty is a fine and imprisonment of up to five years. The federal FCA has been an effective enforcement tool for the federal government and many states have enacted similar false claims acts as well.
The Company seeks to structure its arrangements with physicians and other clients to be in compliance with the AKS, Stark Law, state laws, and the FCA and to stay abreast of current developments and changes in the law and regulations. However, these laws and regulations are complex and subject to interpretation. Consequently, we are unable to ascertain with certainty
15
| NEOGENOMICS, INC. | ||||||||
that our arrangements and transactions will not be subject to scrutiny and, if scrutinized, will not result in sanctions or penalties. The Company has taken, and will continue to take, actions, including robust auditing and monitoring activities, to endeavor to ensure compliance with the myriad federal and state laws that govern our business.
Medicare Payment Guidelines
We have various billing arrangements with our clients and with third party payers, including the Medicare program. When the Company bills the client for all, or a portion of, a laboratory test performed, we believe these client billing arrangements are priced competitively at fair market value. These client billing arrangements may implicate the Medicare program’s prohibition against charging the Medicare or Medicaid programs fees substantially in excess of the Company’s usual and customary charges. Given our participation in Medicare and Medicaid, we are subject to Medicare and Medicaid regulations related to billing those programs as well as agency subregulatory guidance regarding the same, the federal Stark Law, federal and state anti-kickback statutes, and the federal FCA and state equivalents.
In light of the various federal regulations and guidance from the OIG, the Company seeks to price its products competitively while endeavoring to meet applicable statutes and regulations.
Environmental Health and Safety
The Company is subject to licensing and regulation under federal and state laws relating to the protection of the environment as well as human health and safety laws and regulations relating to the handling, transportation, and disposal of medical specimens, hazardous materials, and infectious and hazardous waste. Company laboratories are subject to applicable laws and regulations, primarily at the state level, relating to the management and disposal of regulated medical wastes, including laboratory specimens, and the Company generally utilizes outside vendors for disposal of such waste materials. In addition to its comprehensive regulation of health and safety in the workplace in general, the Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for healthcare employers, including clinical laboratories and other healthcare-related facilities, whose workers may be exposed to chemical hazards as well as biological, physical and safety hazards, including blood-borne pathogens such as HIV and hepatitis B and C viruses. These regulations, among other things, require work practice controls, personal protective clothing and equipment, training, medical follow-up, vaccinations, and other measures designed to minimize and mitigate exposure to, and transmission of, blood-borne pathogens and other types of hazards. For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following agencies: the U.S. Department of Transportation, the U.S. Public Health Service, the U.S. Postal Service, the Office of Foreign Assets Control, and the International Air Transport Association. Other countries where the Company conducts business have similar laws and regulations concerning the environment and human health and safety with which the Company must also comply. The Company seeks to comply with all relevant environmental and human health and safety laws and regulations. Failure to comply could subject the Company to various administrative and/or other enforcement actions.
Confidentiality and Security of Personal Information
The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) contains provisions that protect individually identifiable health information from unauthorized use or disclosure by covered entities and their business associates. The Office for Civil Rights of HHS (“OCR”), the agency responsible for enforcing HIPAA, has published regulations to address the privacy (the “Privacy Rule”) and security (the “Security Rule”) of protected health information (“PHI”) and notification of breaches of PHI (the “Breach Notification Rule,” and, collectively, the “HIPAA Rules”). The Company acts as a covered entity under HIPAA and has adopted policies and procedures designed to comply with HIPAA, including the HIPAA Rules. Many of the health care facilities and providers that refer specimens to the Company are also bound by HIPAA. HIPAA additionally requires that all providers that transmit claims for health care goods or services electronically utilize standard transaction and data sets and use standardized national provider identification codes. We believe that the Company has taken necessary steps to comply with HIPAA regulations. For example, the Company utilizes standard transaction data sets, and has obtained and implemented national provider identifiers, or NPIs, as the standard unique health identifier in filing and processing health care claims and other transactions. HIPAA violations may be subject to criminal and civil penalties.
The Health Information Technology for Economic and Clinical Health Act (“HITECH Act”), enacted as part of the American Recovery and Reinvestment Act (“ARRA”), extended the scope of HIPAA to permit enforcement against business associates, which are entities that use PHI to provide certain services on behalf of covered entities, for HIPAA for violations. The HITECH Act also established new requirements to notify the OCR of a breach of PHI, and allows the state Attorneys General to bring actions to enforce violations of HIPAA. In certain circumstances, we act as a business associate under HIPAA and could be subject to such enforcement if we were to fail to comply with HIPAA as a business associate.
16
| NEOGENOMICS, INC. | ||||||||
In addition to the HIPAA Rules described above, the Company is subject to additional federal and state laws regarding the handling and disclosure of patient records and patient health information. Effective April 5, 2021, HHS published a final rule implementing the information blocking provisions (“Information Blocking Rules”) of the 21st Century Cures Act. The Information Blocking Rules prohibit covered actors, including healthcare providers, from engaging in activity that is likely to interfere with the access, exchanges, or use of electronic health information (“EHI”) unless such activity falls into one of eight exceptions. The Information Blocking Rules provide for civil monetary penalties for noncompliance by healthcare IT vendors and, separately, “appropriate disincentives” for noncompliance by healthcare providers. The HIPAA Rules do not supersede state laws that may be more stringent; therefore, we are required to comply with both federal privacy and security regulations as well as varying state privacy and security laws and regulations. These laws vary widely. For example, many states have implemented genetic testing and privacy laws imposing specific patient consent requirements and limiting the disclosure of genetic test results. Penalties for violation of state laws can include sanctions against a laboratory’s licensure as well as civil or criminal penalties. Additionally, private individuals may have a right of action against the Company for violations of a state’s privacy laws. We believe that we are in material compliance with current state laws regarding the confidentiality of health information, and we will continue to monitor and comply with new or changing state laws.
Further, we are subject to certain comprehensive state laws governing the processing of personal information. In particular, the California Consumer Privacy Act (“CCPA”) took effect on January 1, 2020, and imposed privacy compliance obligations with regard to the personal information of California residents. This legislation created significant new requirements for identifying, managing, securing, tracking, producing, and deleting consumer personal information and granted new rights to California residents, including the right to opt out of their data being sold to a third party by the Company. The CCPA defines personal information extremely broadly as “information that identifies, relates to, describes, is capable of being associated with, or could reasonably be linked, directly or indirectly, with a particular consumer or household.” Like the international privacy laws discussed below, this creates greater complexity in implementing a compliance program to support these requirements. The CCPA law became enforceable by the California Attorney General on July 1, 2020, and the Company has implemented significant mechanisms to promote compliance with this law. The CCPA’s protections have been expanded by the California Privacy Rights Act (“CPRA”), which became operational in most key respects on January 1, 2023. Similar laws have been proposed or passed at the U.S. federal and state level, including the Virginia Consumer Data Protection Act, which took effect on January 1, 2023, the Colorado Consumer Protection Act, which took effect on July 1, 2023, the Connecticut Data Privacy Act, which took effect on July 1, 2023, and the Utah Consumer Privacy Act, which took effect on December 31, 2023. We expect that other states will enact similar legislation in the future, and we will be required to analyze the effect of each of these laws on our business.
Due to the Company’s international expansion, we are subject to a variety of international laws which serve to protect the personal data of individuals who are located in those countries. These laws include the European Union’s General Data Protection Regulation (“GDPR”), the United Kingdom GDPR, and similar privacy laws in other jurisdictions. These laws cover a broader range of data in addition to patient data including data of employees, clients, and other individuals whose data we hold. Like HIPAA, these laws contain regulatory requirements for both robust data privacy and security programs and require data breach reporting should personal data be used or disclosed in a manner not allowed under the laws. Penalties for violations of these laws can be significant; for instance, GDPR’s maximum penalties are up to the greater of 4.0% of a company’s annual global turnover or €20.0 million. Although the Company’s business is conducted primarily in the United States, we do receive some samples for clinical testing from countries outside of the United States, have employee data from European Union and the United Kingdom and we collect data of individuals internationally as part of the Company’s Advanced Diagnostics business, which obligates us to comply with these laws. We have developed privacy and security programs intended to meet these international obligations and continue to reassess and improve these programs continually.
Available Information
Our internet website address is www.neogenomics.com. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), are available free of charge through our website as soon as reasonably practicable after we electronically file with or furnish them to the SEC and are available in print to any stockholder who requests a copy. Please note that our website address is provided in this Annual Report on Form 10-K as an inactive textual reference only. Information on our website shall not be deemed incorporated into, or to be part of, this Annual Report on Form 10-K.
Additionally, the SEC maintains a website that contains reports, proxy statements, information statements, and other information regarding issuers, including us, that file electronically with the SEC at www.sec.gov.
17
| NEOGENOMICS, INC. | ||||||||
ITEM 1A. RISK FACTORS
We are subject to various risks that may materially harm our business, financial condition, and results of operations. They are not, however, the only risks we face. Additional risks and uncertainties not presently known to us or that we currently believe not to be material may also adversely affect our business, financial condition, or results of operations. An investor should carefully consider the risks and uncertainties described below and the other information in this filing before deciding to purchase our common stock. If any of these risks or uncertainties actually occurs, our business, financial condition, or operating results could be materially harmed. In that case, the trading price of our common stock could decline or we may be forced to cease operations.
Risk Factors Summary
The following is a summary of the material risks that could adversely affect our business, financial condition or results of operations.
Risks Relating to Our Business
•Our business is subject to rapid scientific change, which could have a material adverse effect on our business, results of operations, and financial condition.
•We face the risk of capacity constraints, which could have a material adverse effect on our business, results of operations, and financial condition.
•Increased competition, including price competition, could have a material adverse impact on our net revenues and profitability.
•New product development and commercialization involve a lengthy and complex process and we may be unable to develop or commercialize new products on a timely basis, or at all.
•Failure to develop, or acquire licenses for, new or improved testing technologies could materially and adversely affect our revenues.
•The potential loss or delay of our material Advanced Diagnostics customer contracts or of multiple contracts could adversely affect our results.
•We may become involved in litigation that may materially adversely affect us.
•Intellectual property dispute over the RaDaR® assay may necessitate redesign, licensing, discontinuation, or significant damages, potentially harming our overall financial condition, results of operations, or cash flows.
•Our involvement with clinical trials and research services create a risk of liability.
•Our investments in marketable securities are subject to certain risks which could affect our overall financial condition, results of operations, or cash flows.
•Other manufacturers may discontinue or recall testing products used in our business.
•We depend substantially upon third parties for payment of services, which reliance could have a material adverse effect on our cash flows and results of operations.
•We may fail to protect our facilities, which could have a material adverse effect on our business, results of operations, and financial condition.
•We depend on information technology systems and maintain protected personal data, and a cyber-attack or other breach affecting these information technology systems or protected data could have a material adverse effect on our results of operations.
•Performance issues, service interruptions, or price increases by our shipping carriers could adversely affect our business, results of operations, and financial condition, and harm our reputation and ability to provide our specialized diagnostic services on a timely basis.
•We use biological and hazardous materials that require considerable expertise and expense for handling, storage, or disposal and may result in claims against us.
Risks Related to Our Common Stock and Indebtedness
•The price of our common stock may fluctuate significantly.
•Servicing our Convertible Notes require a significant amount of cash. We may not have sufficient cash flow from our business to pay our obligations under the Convertible Notes, which could adversely affect our financial condition and operating results.
18
| NEOGENOMICS, INC. | ||||||||
•We may not have the ability to raise the funds necessary to settle conversions of the Convertible Notes in cash or to repurchase the Convertible Notes upon a fundamental change, and our future debt may contain limitations on our ability to pay cash upon conversion or repurchase of the Convertible Notes.
•The capped call transactions may affect the value of the 2028 Convertible Notes and our common stock.
•Conversion of the Convertible Notes may dilute the ownership interest of existing stockholders or may otherwise depress the price of our common stock.
Risks Relating to Government Regulation and Reimbursement
•If the FDA were to begin to enforce regulation of Laboratory Developed Tests it could require us to conduct additional clinical trials, result in increased costs or delays, or we could fail to obtain necessary regulatory approvals, all of which could harm our business.
•Healthcare reform efforts may impact our business and the pricing we receive for our services.
•Changes in laws, regulations, contracting arrangements with payers, or payer policies, including steps taken by payers to control utilization and reimbursement of healthcare services, may adversely affect coverage or reimbursement for our specialized diagnostic services, which may decrease our revenues and adversely affect our results of operations and financial condition.
•Failure to comply with laws and regulations regarding laboratory licensing and operations, including CLIA environmental, health, and safety laws and regulations such as the federal Occupational Safety and Health Administration Act and the Needlestick Safety and Prevention Act, could result in fines and penalties and loss of licensure, and have a material adverse effect upon our business.
•Our net revenue will be diminished if payers do not adequately cover or reimburse our services.
•Our operations are subject to strict laws prohibiting fraudulent billing and other abuse, and our failure to comply with such laws could result in substantial penalties, including exclusion from participation in Medicare, Medicaid, and other governmental payer programs.
•The failure to comply with fraud and abuse laws, including physician self-referral laws and anti-kickback laws, may subject us to liability, penalties, or limitation of operations.
•Failure to comply with federal, state and international laws related to privacy and security could result in fines, penalties, and damage to the Company’s reputation with customers and could have a material adverse effect upon the Company’s business.
General Risk Factors
•We are dependent on key personnel and need to hire additional qualified personnel in order for our business to succeed.
•We may not be able to implement our business strategy, which could impair our ability to continue operations.
•We may be unable to realize estimated benefits from our cost reduction and restructuring efforts and our profitability may be hurt or our business might otherwise be adversely affected.
•If we are unable to successfully integrate future acquisitions with our legacy business, the anticipated benefits of such transaction may not be realized.
•If goodwill and intangible assets that we recorded in connection with our acquisitions become impaired, we may have to take significant charges against earnings.
•We may incur greater costs than anticipated, which could result in sustained losses.
•We may face fluctuations in our results of operations and we are subject to seasonality in our business which could negatively affect our business operations.
•The steps we have taken to protect our proprietary rights may not be adequate, which could result in infringement or misappropriation by third parties.
Risks Relating to Our Business
If we are unable to keep pace with the rapid scientific and technological change characteristic to our industry, or to develop, or acquire licenses for, new or improved testing technologies, our competitive position, business, results of operations, and financial condition could be harmed.
The market for genetic and molecular testing services is characterized by rapid scientific developments, evolving industry standards and customer demands, and frequent new product introductions and enhancements. For example, new tests
19
| NEOGENOMICS, INC. | ||||||||
developed by our competitors may prove superior and replace our existing tests. Additionally, certain technological changes, such as advances in point-of-care testing, could reduce the need for the laboratory tests we provide. Our future success will depend in significant part on our ability to continually improve our offerings in response to both evolving demands of the marketplace and competitive service offerings. If we are unsuccessful in keeping pace with scientific and technological changes, or enhancing our products to meet evolving industry standards or developing customer demands, our competitive position, business, results of operations, and financial condition may be materially and adversely affected.
In addition, other companies or individuals, including our competitors, may obtain patents or other intellectual property rights that would prevent, limit or interfere with our ability to develop, perform or sell our solutions or operate our business or increase our costs. In addition, they could introduce new tests, technologies or services that may result in a decrease in the demand for our services or cause us to reduce the prices of our services. Our success will depend, in part, on our ability to develop, acquire or license new and improved technologies on favorable terms and to obtain appropriate coverage and reimbursement for these technologies. We may not be able to negotiate acceptable licensing arrangements, and we cannot be certain that such arrangements will yield commercially successful diagnostic tests. If we are unable to license these testing methods at competitive rates, our research and development costs may increase as a result. In addition, if we are unable to license new or improved technologies to expand our testing operations, our testing methods may become outdated when compared with our competition and testing volume and revenue may be materially and adversely affected.
We face the risk of capacity constraints, which could have a material adverse effect on our business, results of operations, and financial condition.
We compete in the market place primarily on three factors: (i) the quality and accuracy of our test results; (ii) the speed or turnaround times of our testing services; and (iii) our ability to provide after-test support to those physicians requesting consultation. Any unforeseen increase in the volume of clients could strain the capacity of our personnel and systems, leading to unacceptable turnaround times or customer service failures. In addition, as the number of our clients and specimens increases, our products, services, and infrastructure may not be able to scale accordingly. We may also not be able to hire additional licensed medical technologists that we need to handle increased volumes. Any failure to handle higher demand for our products and services could lead to the loss of established clients or could otherwise cause our clients to choose not to use us in the future, which could severely harm our business, results of operations, and financial condition. In addition, based on the importance of the subject matter of our tests, inaccurate results could result in improper treatment of patients and potential liability for us.
Increased competition, including price competition, could have a material adverse impact on our net revenues and profitability.
The market for genetic and molecular testing services is highly competitive, and, given the opportunities in this market within the laboratory testing industry, we expect competition to continue increasing. Our competitors within the broader genomics profiling space include laboratory companies such as Quest Diagnostics, Laboratory Corporation of America, and Bio-Reference Laboratories. These are large national laboratories that possess greater name recognition, larger customer bases, and significantly greater financial resources and employ substantially more personnel than we do. We also face increased competition from laboratories that are more specialized and focused on particular areas such as liquid biopsies or large tissue based molecular panels such as Guardant Health, Inc., Natera, Inc., Exact Sciences Corp, Caris Life Science, Tempus Labs, Inc and Myriad Genetics, Inc. Our competitors may develop products and services that are superior to ours or that achieve greater market acceptance than our offerings. Many of our competitors have long established relationships with their customers and third-party payers. We cannot assure you that we will be able to compete successfully with these entities or other competitors in the future.
The laboratory testing business is intensely competitive, both in terms of price and service. Pricing of laboratory testing services is often one of the most significant factors used by healthcare providers and third-party payers in selecting a laboratory. As a result of the laboratory testing industry undergoing consolidation, larger laboratory providers are able to increase cost efficiencies afforded by large-scale automated testing. This consolidation results in greater price competition. We may be unable to increase cost efficiencies sufficiently, if at all, and as a result, our net earnings and cash flows could be negatively impacted by such price competition. Additionally, we may also face changes in fee schedules, competitive bidding for laboratory services, or other actions or pressures reducing payment schedules as a result of increased or additional competition. Furthermore, many competitors are developing information technology-based tools to support the integration of next-generation sequencing testing into the clinical setting. These companies may also use their own tests or others to develop an integrated system which could limit our access to certain networks. See Part I, Item 1, “Business” in this Annual Report on Form 10-K for additional information about our competitors and competitive position.
20
| NEOGENOMICS, INC. | ||||||||
Also, in each of these markets, consolidation in our actual or potential customer base results in increased competition for important market segments and fewer available customers. Consolidation among healthcare providers and the formation of buying groups have put pressure on pricing and sales of our products, and in some instances, required payment of fees to group purchasing organizations. Our success in these areas depends partly on our ability to enter into contracts with integrated health networks and group purchasing organizations. If we are unable to enter into contracts with these group purchasing organizations and integrated health networks on terms acceptable to us, our sales and results of operations may be adversely affected. Even if we are able to enter into these contracts, they may be on terms that negatively affect our current or future profitability. As a result of this and future consolidations, our customer diversity may decrease and our business may be adversely affected.
New product development and commercialization involve a lengthy and complex process and we may be unable to develop or commercialize new products on a timely basis, or at all.
Our success depends on our ability to develop new tests and other related products while improving the performance, cost-effectiveness and timeliness of our existing products. Our products that are under development have taken time and considerable resources to develop, and we may not be able to complete the development and commercialization of such products for clinical use on a timely basis, or at all. For example, there can be no assurance that we will be able to produce commercial products for early detection of cancer. Before we can commercialize any new products, we will need to expend significant funds in order to:
•conduct substantial research and development, including validation studies and clinical studies;
•further develop and scale our laboratory processes to accommodate different products, including the expansion of our medical staff and PhDs; and
•further develop and scale our infrastructure to be able to analyze increasingly large amounts of data; and
•seek and obtain regulatory clearance or approvals of our new products, as required by applicable regulations.
Our product development process involves a high degree of risk, and product development efforts may fail for many reasons, including:
•failure of the product to perform as expected, including defects and errors;
•lack of validation data; or
•failure to demonstrate the clinical utility of the product.
As we develop products, we have made and will have to make significant investments in product development, marketing and selling resources, including investing heavily in clinical studies, which could adversely affect our future cash flows.
We expect to make significant investments in the development of new genetic tests and other future products. New product development and commercialization involve a lengthy and complex process, and we may be unable to develop or commercialize new products on a timely basis, or at all.
We are seeking to develop new proprietary and non-proprietary genetic tests and to build a pipeline for future products and services. Products that are under development have taken time and considerable resources to develop, and we may not be able to complete the development and commercialization of such products on a timely basis, or at all. For example, there can be no assurance that we will be able to produce commercial products for CGP or MRD. Before we can commercialize any new products, we will need to expend significant funds in order to conduct substantial research and development, including validation studies and clinical studies and to further develop and scale our infrastructure and marketing capabilities.
Our product development process involves a high degree of risk, and product development efforts may fail for many reasons, including:
•failure of product to perform as expected, including defects and errors;
•lack of validation data; or
•failure to demonstrate the clinical utility of the product.
As addressed in Part I, Item 1, “Business— Licensure, Accreditation, and Quality Standards” in this Annual Report on Form 10-K, we cannot be certain as to which of our tests, if any, would require FDA approval or clearance under any of the proposed regulatory frameworks for LDTs and, if required, that our tests could obtain such approval or clearance. Even if the FDA and other regulatory authorities clear or approve a new product or service we develop, we would need to commit substantial resources to commercialize, sell and market it before it could be profitable, and the product or service may never be commercially viable. In developing a test, we must make numerous assumptions, often many years before a test is ready
21
| NEOGENOMICS, INC. | ||||||||
for use, regarding the commercial viability of a test, including with respect to our customers’ interest in a test, payers’ willingness to pay for a test, our costs to perform a test, and availability and attractiveness of competing offerings. As a result, it is possible that we may introduce a new product that uses technologies or methods of analysis that have been displaced by the time of launch, competes with one or more of our other products, addresses an opportunity that no longer exists or is smaller than anticipated, or produces data that provides less utility to our customers than anticipated or otherwise is not competitive at the time of launch. The expenses or losses associated with unsuccessful product development or launch activities, or a lack of market acceptance of our new products or services, could adversely affect our business, financial condition or results of operations.
The potential loss or delay of our material Advanced Diagnostics customer contracts or of multiple contracts could adversely affect our results.
The revenue attributable to our Advanced Diagnostics clients may also fluctuate in the future, which could have an adverse effect on our financial condition and results of operations. Most of our Advanced Diagnostics segment clients can terminate our contracts without cause upon proper notice, and we experience termination or non-renewal of our Advanced Diagnostics contracts in the ordinary course of business. Our Advanced Diagnostics clients may delay, terminate or reduce the scope of our contracts for a variety of reasons beyond our control, including but not limited to actions by regulatory authorities, negative clinical results, lack of patient enrollment, lack of available financing or shifts in internal priorities. In addition, adverse speculation about our existing or potential relationships with our Advanced Diagnostics clients may be a catalyst for adverse speculation about us, our products and our technology, which can adversely affect our reputation and business. Delays, terminations or reductions in the scope of our contracts impact our ability to convert our backlog into revenue for the Company. Our ability to realize the full benefits of our backlog of contractually committed services due to delay, cancellation or reduction in our client’s contractual commitments, would materially impact our revenues. In addition, the terminability of our contracts puts increased pressure on our quality control efforts, since not only can our contracts be terminated by clients as a result of poor performance, but any such termination may also affect our ability to obtain future contracts from the clients involved and others.
We may become involved in litigation, and our insurance may not sufficiently cover all claims brought against us, which will increase our expenses and may adversely affect our business and results of operations.
From time to time, we may become involved in various legal proceedings relating to matters incidental to the ordinary course of our business, including employment, commercial, product liability, class action, whistleblower and other litigation and claims, and governmental and other regulatory investigations and proceedings. For example, development, marketing, sale, and performance of laboratory testing services expose us to the risk of litigation, including professional negligence or product liability claims, were someone to allege that our tests failed to perform as designed. We may also be subject to liability for errors in the test results we provide to pathologists and oncologists or for a misunderstanding of, or inappropriate reliance upon, the information we provide. Such matters and other litigation against us can be time-consuming, divert management's attention and resources, cause us to incur significant expenses or liability and/or require us to change our business practices. In addition, damages assessed in connection with, and the costs of defending, any legal action could be substantial. Because of the potential risks, expenses, and uncertainties of litigation, we may, from time to time, settle disputes, even where we believe that we have meritorious claims or defenses. We also may be faced with litigation claims that exceed our insurance coverage or are not covered under any of our insurance policies. In addition, litigation could have a material adverse effect on our business if it impacts our existing and potential customer relationships, creates adverse public relations, diverts management resources from the operation of the business, or hampers our ability to otherwise conduct our business. Because litigation is inherently unpredictable, we cannot assure you that the results of any of these actions will not have a material adverse effect on our business, results of operations and financial condition.
One of our competitors has alleged that our RaDaR® assay and certain tests are infringing on its intellectual property, and we may be required to redesign the technology, obtain a license, cease using the RaDaR® assay altogether and/or pay significant damages, among other consequences, any of which may have a material adverse effect on our business as well as our financial condition and results of operations.
One of our competitors, Natera, Inc., or Natera, filed a complaint against NeoGenomics Laboratories, Inc. alleging our RaDaR® assay and multiplex PCR of at least 25 cancer related targets from cell-free DNA infringe on certain of Natera’s U.S. patents. Additionally, Natera filed a motion for a preliminary injunction hearing on July 31, 2023 seeking to enjoin the Company from selling the RaDaR® assay. A preliminary injunction hearing occurred on November 27, 2023 and on December 27, 2023, the court granted Natera’s preliminary injunction on the basis of a likelihood of infringement of a Natera patent. We may continue to make, use, and sell the RaDaR® assay solely for continued use of the RaDaR® assay: (i) for those patients already using it before the entry of this injunction, (ii) in support of research and development with other persons or
22
| NEOGENOMICS, INC. | ||||||||
entities on projects or studies that began before the entry of this injunction, or (iii) for use in or in support of clinical trials in process or already approved by an agency of the United States. Natera posted a $10 million bond with the court on January 12, 2024. On December 28, 2023, NeoGenomics appealed the preliminary injunction to the Federal Circuit. The appeal was docketed at the Federal Circuit on January 4, 2024. On February 5, 2024, NeoGenomics filed an Emergency Motion to Stay the Preliminary Injunction pending Appeal and a Motion to Expedite the appeal. The Federal Circuit granted expedited briefing of the appeal with oral arguments scheduled for March 29, 2024. Separately, the court proceedings on the patent infringement claims are in the discovery stage.
If our RaDaR® assay is found to infringe any of Natera's patents, we could be required to redesign our technology or obtain a license from Natera to continue developing, manufacturing, marketing, selling and commercializing the RaDaR® assay and related products. However, we may not be successful in the redesign of its technology or able to obtain any such license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving Natera and other third parties the right to use the same technologies licensed to us, and Natera could require us to make substantial licensing, royalty and other payments. We also could be forced, including by court order, to permanently cease developing, manufacturing, marketing and commercializing our products that are found to be infringing. In addition, we could be found liable for significant monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed Natera's asserted patents. Even if we were ultimately to prevail, litigation with Natera could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
We cannot reasonably estimate the final outcome, including any potential liability or any range of potential future charges associated with these litigations. However, any finding of infringement by us of Natera's asserted patents may have a material adverse effect on our business, as well as our financial condition and results of operations.
Our involvement with clinical trials and research services create a risk of liability.
We have conducted clinical trials and presently support many clinical trials run by third parties, which ordinarily involve testing an investigational drug on a limited number of individuals to evaluate the drug’s safety, determine a safe dosage range and identify side effects. Errors or omissions could occur during a clinical trial that may result in harm to study volunteers, or if unnoticed and regulatory approval is received, to consumers of the drug, or that may undermine the usefulness of the clinical trial or data from the clinical trial and may delay the entry of a drug to the market. In addition, failure to operate such clinical trials in accordance with the FDA, the U.S. Drug Enforcement Agency (“DEA”), and other applicable regulations could result in disruptions to our operations.
Our contracts with the pharmaceutical sponsors include provisions entitling us to be indemnified or entitling us to a limitation of liability. These provisions do not uniformly protect us against liability arising from certain of our own actions or those of our professional staff, such as gross negligence or misconduct. We could be materially and adversely affected if we were required to pay damages or bear the costs of defending any claim which is not covered by or exceeds the limits of a contractual indemnification provision, or in the event that a party who must indemnify us does not fulfill its indemnification obligations, or which is beyond the level of our insurance coverage.
Our investments in marketable securities are subject to certain risks which could affect our overall financial condition, results of operations, or cash flows.
We invest a portion of our available cash and cash equivalents by purchasing marketable securities in a managed portfolio and direct investments in a variety of debt securities, including U.S. Treasury securities and corporate debt securities. The primary objective of our investment activity is to maintain the safety of principal while maximizing yields without significantly increasing risk. Should any of our investments or marketable securities lose value or have their liquidity impaired, it could affect our overall financial condition. Additionally, if we choose to, or are required to, sell these securities in the future at a loss, our consolidated operating results or cash flows may be affected.
Other manufacturers may discontinue or recall testing products used in our business.
We rely heavily on reagents, test kits and instruments manufactured by third parties in our testing services. From time to time, manufacturers have discontinued or recalled, and may in the future discontinue or recall, the reagents, test kits or instruments used by us to perform laboratory testing. Such discontinuations or recalls could adversely affect our costs, testing volume and revenues. We have had certain tests discontinued by manufacturers and have had to develop alternative solutions for our clients.
We depend substantially upon third parties for payment of services, which reliance could have a material adverse effect on our cash flows and results of operations.
23
| NEOGENOMICS, INC. | ||||||||
Our business consists of clinical laboratories that provide medical testing services for doctors, hospitals, and other laboratories on patient specimens that are sent to our laboratories. In the case of some specimen referrals that are received for patients that are not in-patients or out-patients at a hospital or institution or otherwise sent by another reference laboratory, we typically bill the patient’s insurance company or a government program for our services. As such, we rely on the cooperation of numerous third-party payers, including but not limited to Medicare, Medicaid, and various insurance companies, to get paid for performing services on behalf of our clients and their patients. The amount of such third-party payments is governed by contractual relationships in cases where we are a participating provider for a specified insurance company or by established government reimbursement rates in cases where we are an approved provider for a government program such as Medicare or Medicaid. However, we do not have contractual relationships with some of the insurance companies with whom we deal, nor are we necessarily able to become an approved provider for all government programs. In such cases, we are deemed to be a non-participating provider, and there is no contractual assurance that we will be able to collect the amounts billed to such insurance companies or government programs. Until such time we become a participating provider with such insurance companies, there can be no contractual assurance that we will be paid for the services we bill to such insurance companies or patients, and such third parties may change their reimbursement policies for non-participating providers in a manner that may have a material adverse effect on our cash flow or results of operations. When new Current Procedural Terminology (“CPT”) codes are introduced by the American Medical Association (“AMA”) it often takes time for commercial insurance providers to recognize the new codes, which can significantly impact the timing of payments, if any, and can increase our days-sales-outstanding. Medicare has also, at times, issued codes or coding guidance that conflicts with the AMA CPT coding, which can cause confusion when secondary insurance is involved. Insurance companies may also try to steer business away from us towards in-network providers by sending letters to physicians and even imposing financial penalties if they continue to send us business. Additionally, due to the fluctuating and uncertain nature of the reimbursement environment, including the amount that payers reimburse us for any of our services, we estimate the amount of revenue to be recognized at the time services are provided and record revenue adjustments if and when the cash subsequently received for the services differs from the revenue recorded. Due to this inherently uncertain nature of the reimbursement landscape, previously recorded revenue adjustments are not indicative of future revenue adjustments from actual cash collections, which may fluctuate significantly.
If our facilities become damaged or inoperable due to disasters, power loss, break-ins or similar events, we may be unable to continue our operations or our services could be interrupted or delayed, which could have a material adverse effect on our business, results of operations, and financial condition.
Our operations are dependent in part upon our ability to protect our laboratory operations, including our information technology systems, against physical damage from natural or man-made disasters, such as explosions, fire, floods, hurricanes, earthquakes, power loss, telecommunications failures, break-ins, public health issues, epidemics or pandemics, terrorist attacks, and similar events beyond our control. We do not presently have an emergency back-up generator in place at our Tampa, Florida, Nashville, Tennessee, Atlanta, Georgia, or Phoenix, Arizona laboratory locations, which would otherwise mitigate to some extent the effects of a prolonged power outage. The occurrence of any of these events could result in interruptions, delays, or cessations in service to clients, which could have a material adverse effect on our business, results of operations, and financial condition.
We depend on our information technology systems and those of our third-party service providers and maintain protected personal data, and a cyber-attack or other breach affecting these information technology systems or protected data could have a material adverse effect on our business, reputation and results of operations.
Our laboratory operations depend, in part, on the continued performance of our information technology systems as well as those of our third-party service providers. Our information technology systems are susceptible to a cyber-attack, malicious intrusion, breakdown, destruction, loss of confidential information or data (including credit card and other financial information), or other significant disruption. These systems have been and are expected to continue to be the target of malware and other cyber-attacks. The continued hybrid working environment following the COVID-19 pandemic has further increased the risk of cyber-attacks and other cybersecurity risks faced by us and our third-party service providers due to our reliance on the internet technology and the number of our employees who are working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. In addition, third-party hacking attempts may cause our information technology systems and related products, protected data, or proprietary information to be compromised or stolen. A significant attack or other disruption could result in adverse consequences, including increased costs and expenses, manufacturing challenges or disruption, problems with product functionality, damage to customer relations, lost revenue, and legal or regulatory penalties. Sustained system failures or interruption of our systems in one or more of our laboratory operations could disrupt our ability to process laboratory requisitions, perform testing, provide test results in a timely manner, and/or bill the appropriate party.
24
| NEOGENOMICS, INC. | ||||||||
We also rely on the information technology systems of our third-party service providers for information technology services and application hosting. Their systems are also vulnerable to attack and damage or interruption from telecommunications or network failures, natural disasters, employee theft or misuse, human error, fraud, denial, or degradation of service attacks, sophisticated nation-state and nation-state supported actors or unauthorized access or misuse. Despite any security barriers implemented by these third parties to protect against such threats, which are largely beyond our control, the information technology systems of our third-party service providers may be compromised resulting in potential disruption of their services or loss of business information (including our proprietary and confidential information) stored by these third parties.
We also collect, manage and process sensitive data, including protected health information subject to HIPAA and genetic information, in connection with the operation of our business and our service offerings. Breaches resulting in the loss or unauthorized access to or use of such information, including that of our employees, could result in violations of HIPAA, the HITECH Act, GDPR, and other federal, state, and international laws regarding the privacy, confidentiality, and security of such information. A breach of this protected information could result in adverse consequences, including regulatory inquiries or litigation, increased costs and expenses, including costs related to insurance and remediation of any security vulnerabilities, reputational damage, lost revenue, and fines or penalties.In addition, we collect and store intellectual property and proprietary business information owned or controlled by us or other third parties for our customers and payers. Cyber-attacks, security breaches, computer viruses, malware and other incidents could cause misappropriation, loss or other unauthorized disclosure of such information. Increasingly complex methods have been used in cyber-attacks, including ransomware, phishing, structured query language injections, social engineering schemes, and distributed denial-of-service attacks. A cyber-attack can also be in the form of unauthorized access or a blocking of authorized access. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased.
While we invest in our systems and technology and in the protection of our products and data to reduce the risk of an attack or other significant disruption, there can be no assurance that these measures and efforts will prevent future attacks or other significant disruptions to any of the systems on which we rely. Similarly, there can be no assurance that third party information technology providers with whom we contract will not suffer a significant attack or disruption that impacts customers, such as supply chain attacks. Any significant breach, attack, disruption, or failure of our information technology systems could adversely affect our business, results of operations, and financial condition.
Performance issues, service interruptions, or price increases by our shipping carrier could adversely affect our business, results of operations, and financial condition, and harm our reputation and ability to provide our specialized diagnostic services on a timely basis.
Expedited, reliable shipping is essential to our operations. One of our marketing strategies principally highlights the reliability of our point-to-point transport of patient samples. We rely heavily on a single provider of transport services, FedEx Corporation (the “Carrier”), for reliable and secure point-to-point transport of patient samples to our laboratory and enhanced tracking of these patient samples. Should the Carrier encounter delivery performance issues such as loss, damage, or destruction of a sample, it may be difficult to replace our patient samples in a timely manner and such occurrences may damage our reputation and lead to decreased demand for our services and increased cost and expense to our business. In addition, any significant increase in shipping rates could adversely affect our operating margins and results of operations. Similarly, strikes, severe weather, natural disasters, or other service interruptions by delivery services we use would adversely affect our ability to receive and process patient samples on a timely basis and, accordingly, our ability to compete with other providers of similar services. If the Carrier or we were to terminate our relationship, we would be required to find another party to provide expedited, reliable point-to-point transport of our patient samples. There are only a few other providers of such nationwide transport services, and there can be no assurance that we will be able to enter into arrangements with another provider on acceptable terms, if at all. Finding a new provider of transport services would be time-consuming and costly and result in delays in our ability to provide our specialized diagnostic services. Even if we were to enter into an arrangement with such alternative provider, there can be no assurance that they will provide the same level of quality in transport services currently provided to us by the Carrier. If the new provider does not provide the required quality and reliable transport services, it could adversely affect our business, reputation, results of operations, and financial condition.
We use biological and hazardous materials that require considerable expertise and expense for handling, storage, or disposal and may result in claims against us.
We work with hazardous materials, including chemicals, biological agents and compounds, blood samples, and other human tissue that could be dangerous to human health and safety or the environment. Our operations also produce hazardous and biohazardous waste products. We have an Employee Health & Safety Department that closely monitors the use of hazardous materials in our laboratory. Federal, state, and local laws and regulations also govern the use, generation, manufacture,
25
| NEOGENOMICS, INC. | ||||||||
storage, handling, and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair business efforts. If we do not comply with applicable regulations, we may be subject to fines and penalties. In addition, we cannot entirely eliminate the risk of accidental injury or contamination from these materials or wastes to employees and third parties. In the event of contamination or injury, we could be held liable for any resulting damages or penalized with fines, and any liability could exceed our resources. Although we maintain general liability insurance or workers’ compensation insurance policies, such policies and other applicable insurance policies that we maintain may not fully cover any resulting damages and fines arising from biological or hazardous waste.
Risks Related to Our Common Stock and Indebtedness
The price of our common stock has, and may continue to, fluctuate significantly.
The price of our common stock has been, and is likely to continue to be, volatile and it could decline substantially within a short period of time. The price of our common stock could fluctuate significantly for many reasons including the following:
•change in our leadership or Board of Directors;
•future announcements concerning us or our competitors;
•regulatory developments and enforcement actions bearing on advertising, marketing, or sales;
•reports and recommendations of analysts and whether or not we meet the milestones and metrics set forth in such reports;
•gaining or losing large customers or managed care plans;
•introduction of new products or services and related insurance coverage;
•acquisition or loss of significant manufacturers, distributors or suppliers, or an inability to obtain sufficient quantities of materials needed to provide our services;
•quarterly variations in operating results;
•business acquisitions or divestitures;
•changes in the regulation of LDTs;
•changes in governmental or third-party reimbursement practices and rates; and
•fluctuations in the economy, political events, or general market conditions.
In addition, stock markets in general and the market for shares of healthcare stocks in particular, have experienced extreme price and volume fluctuations in recent years, which frequently have been unrelated to the operating performance of the affected companies. These broad market fluctuations may adversely affect the market price of our common stock. In the past, companies that experience volatility in the market price of their securities have often faced securities class action litigation. Whether or not meritorious, litigation brought against us could result in substantial costs, divert our management's attention and resources and harm our ability to grow our business.
Servicing our Convertible Notes requires a significant amount of cash. We may not have sufficient cash flow from our business to pay our obligations under the Convertible Notes, which could adversely affect our financial condition and operating results.
In April 2020, we issued $201.3 million aggregate principal amount of 2025 Convertible Notes, and in January 2021, we issued $345.0 million aggregate principal amount of 2028 Convertible Notes. We may also incur additional indebtedness in the future. Our ability to make scheduled payments of the principal of, pay interest on, or refinance our indebtedness depends on our future performance, which is subject to economic, financial, competitive, and other factors beyond our control. Our business may not continue to generate cash flow from operations in the future sufficient to service our indebtedness and to make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt, or obtaining additional equity capital on terms that may be onerous or highly dilutive. Our ability to refinance the Convertible Notes will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
We may not have the ability to raise the funds necessary to settle conversions of the Convertible Notes in cash or to repurchase the Convertible Notes upon a fundamental change, and our future debt may contain limitations on our ability to pay cash upon conversion or repurchase of the Convertible Notes.
26
| NEOGENOMICS, INC. | ||||||||
Holders of the Convertible Notes have the right to require us to repurchase their Convertible Notes upon the occurrence of a fundamental change at a repurchase price equal to 100% of the principal amount of the Convertible Notes to be repurchased, plus accrued and unpaid interest, if any. In addition, upon conversion of the Convertible Notes, unless we elect to deliver solely shares of our common stock to settle such conversion (other than paying cash in lieu of delivering any fractional share), we will be required to make cash payments in respect of the Convertible Notes being converted. However, we may not have enough available cash or be able to obtain financing at the time we are required to make repurchases of Convertible Notes surrendered therefor or Convertible Notes being converted. In addition, our ability to repurchase the Convertible Notes or to pay cash upon conversions of the Convertible Notes may be limited by law, by regulatory authority, or by agreements governing our future indebtedness. Our failure to repurchase Convertible Notes at a time when the repurchase is required by the respective indenture or to pay any cash payable on future conversions of the Convertible Notes as required by such indenture would constitute a default under the indenture. A default under an indenture or the occurrence of the fundamental change may also lead to a default under agreements governing our future indebtedness. If the repayment of the related indebtedness were to be accelerated after any applicable notice or grace periods, we may not have sufficient funds to repay the indebtedness and repurchase the Convertible Notes or make cash payments upon conversions thereof.
The capped call transactions may affect the value of the 2028 Convertible Notes and our common stock.
In connection with the issuance of the 2028 Convertible Notes, we have entered into capped call transactions with the option counterparties. Upon conversion of any of the 2028 Convertible Notes, we will satisfy our conversion obligation by paying or delivering, as the case may be, cash, shares of our common stock, or a combination of cash and shares of our common stock, at our election, and the capped call transactions are intended to reduce the potential dilution upon conversion of the 2028 Convertible Notes and/or offset some or all of any cash payments we are required to make in excess of the principal amount of converted 2028 Convertible Notes, as the case may be, with such reduction and/or offset subject to a cap.
In connection with these transactions, the option counterparties or their respective affiliates may modify their hedge positions related to the capped call transactions by entering into or unwinding various derivatives with respect to our common stock and/or purchasing or selling our common stock or other securities of ours in secondary market transactions prior to the maturity of the 2028 Convertible Notes (and are likely to do so during any observation period related to a conversion of 2028 Convertible Notes or following any repurchase or redemption of the 2028 Convertible Notes). This activity could also cause or avoid an increase or a decrease in the market price of our common stock or the 2028 Convertible Notes.
Conversion of the Convertible Notes may dilute the ownership interest of existing stockholders or may otherwise depress the price of our common stock.
The conversion of some or all of the Convertible Notes may dilute the ownership interests of existing stockholders to the extent we deliver shares of our common stock upon conversion of any of the Convertible Notes. We have entered into capped call transactions with respect to the 2028 Convertible Notes to reduce the risk of dilution, but to the extent that the conversion price of the 2028 Convertible Notes exceeds the cap price of the capped calls or to the extent that the Convertible Notes are converted, such conversions will dilute the ownership interests of our existing stockholders. The Convertible Notes may from time to time in the future be convertible at the option of their holders prior to their scheduled terms under certain circumstances. Any sales in the public market of the common stock issuable upon such conversion could adversely affect prevailing market price of our common stock. In addition, the existence of the Convertible Notes may encourage short selling by market participants because conversion could be used to satisfy short positions, and the anticipated conversion of the Convertible Notes into shares of our common stock could depress the price of our common stock.
Risks Relating to Government Regulation and Reimbursement
If the FDA were to begin to enforce regulation of Laboratory Developed Tests it could require us to conduct additional clinical trials, result in increased costs or delays, or we could fail to obtain necessary regulatory approvals, all of which could harm our business.
We frequently develop diagnostic tests for clients that cannot currently be provided using test kits approved or cleared by the FDA. Currently, all LDTs are conducted and offered in accordance with the requirements of CLIA and individual state licensing procedures, but the FDA has had a policy of enforcement discretion with regard to LDTs. On September 29, 2023, the FDA published a proposed rule that, if finalized, would end this policy of enforcement discretion for virtually all LDTs in five stages over a four-year period from the date FDA publishes a final rule, and provide for LDTs to be regulated as medical devices. In Phase 1 (effective one year post-finalization), laboratories would be required to comply with medical device (adverse event) reporting and correction and removal reporting requirements. In Phase 2 (effective two years post-finalization), laboratories would be required to comply with all other medical device regulatory requirements (including registration and listing, labeling, and investigational use exemptions), except for quality system and premarket review
27
| NEOGENOMICS, INC. | ||||||||
requirements. In Phase 3 (effective three years post-finalization), laboratories would be required to comply with quality system requirements (i.e., good manufacturing practices). In Phase 4 (effective three and a half years post-finalization, but not before October 1, 2027), laboratories would be required to comply with premarket review requirements for high-risk tests (i.e., tests subject to premarket approval requirements). Finally, in Phase 5 (effective four years post-finalization, but not before April 1, 2028), laboratories would be required to comply with premarket review requirements for moderate- and low-risk tests (i.e., tests subject to de novo or full 510(k) premarket notification requirements). Unlike previous FDA proposals, the proposed rule does not “grandfather” any currently marketed tests.
The content and timing of any final rule on LDTs is uncertain at this time and the FDA has taken a number of prior steps towards regulation of LDTs that have not been implemented. Nevertheless, there is a risk that the FDA’s proposed regulatory process could delay the offering of certain tests and result in additional validation costs and fees. There is also an associated risk that some tests currently offered might become subject to FDA premarket approval or clearance. This FDA approval or clearance process may be time-consuming and costly, with no guarantee of ultimate approval or clearance. If our diagnostic tests are allowed to remain on the market but there is uncertainty about the regulatory status of such tests, if they are labeled investigational by FDA, or if FDA limits our labeling claims, orders or reimbursement may decline.
Congress has also considered a number of legislative proposals in recent years that would amend the regulatory framework for LDTs, including, among other requirements, FDA premarket review of certain LDTs. The most recent such proposal, the VALID Act, was introduced in both the House and Senate on June 24, 2021. The VALID Act was expected to be included in the Omnibus bill signed at the end of 2022, but ultimately was not included. The VALID Act was then reintroduced in March 2023. The bill would subject many LDTs to FDA regulation by creating a new in vitro clinical test, or IVCT, category of regulated products. As proposed, the bill would grandfather many existing LDTs from the proposed premarket approval, quality systems, and labeling requirements, respectively, but would require such tests to comply with other regulatory requirements (including registration and listing and adverse event reporting). To market a high-risk IVCT, reasonable assurance of analytical and clinical validity for the intended use would be needed to be established. Under the VALID Act, a pre-certification process would be established that would allow a laboratory to establish that the facilities, methods, and controls used in the development of its IVCTs meet quality system requirements. If pre-certified, low-risk IVCTs, developed by the laboratory would not be subject to pre-market review. The new regulatory framework would include quality control and post-market reporting requirements. The FDA would have the authority to withdraw approvals for IVCTs for various reasons, including (for example) if there were a reasonable likelihood that the test would cause death or serious adverse health consequences. We cannot predict if the VALID Act (or any other bill) will be enacted in its current (or any other) form. However, it is possible that legislation and resulting FDA regulation may result in increased regulatory burdens and costs for us to seek marketing authorization for and maintain ongoing compliance for our existing tests, any modifications thereto, or any future tests we may develop. If the government begins to regulate our tests, it could require a significant volume of applications, which would be burdensome. Furthermore, governmental bodies could take a long time to review such applications and/or document responses if other laboratories were also required to file applications and/or document responses for each of their LDTs.
In the event that the FDA begins to regulate our tests, it may require additional pre-market clinical testing prior to submitting a premarket approval, premarket notification, or other application to permit commercial sales. Such additional pre-market clinical testing could delay the commencement or completion of clinical testing, significantly increase our test development costs, delay commercialization of any future tests, and interrupt sales of our current tests. Additionally, the results of pre-clinical trials or previous clinical trials may not be predictive of future results, and clinical trials may not satisfy the requirements of the FDA or other non-U.S. regulatory authorities. Many of the factors that may cause or lead to a delay in the commencement or completion of clinical trials may also ultimately lead to delay or denial of regulatory clearance or approval. The commencement of clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites, and the eligibility criteria for the clinical trial. We also cannot be certain that FDA will not enact rules or guidance that could impact our ability to purchase materials necessary for the performance of our LDTs, such as products labeled for research use only. Should any of the reagents we obtain from third party suppliers and use in conducting our LDTs be affected by future regulatory actions, our business could be adversely affected by those actions, including increasing the cost of testing or delaying and limiting or prohibiting the purchase of reagents necessary to perform testing.
We may find it necessary to engage CROs to perform data collection and analysis and other aspects of our clinical trials, which might increase the cost and complexity of our trials. We may also depend on clinical investigators, medical institutions, and CROs to perform the trials. If these parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, or if the quality, completeness, or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or for other reasons, our clinical trials may have to be extended, delayed, or terminated. Many of these factors would be beyond our control. We may not be able to enter into
28
| NEOGENOMICS, INC. | ||||||||
replacement arrangements without undue delays or considerable expenditures. If there are delays in testing or approvals as a result of the failure to perform by third parties, our research and development costs would increase, and we may not be able to obtain regulatory clearance or approval for our tests. In addition, we may not be able to establish or maintain relationships with these parties on favorable terms, if at all. Each of these outcomes would harm our ability to market our tests and/or to achieve sustained profitability.
Healthcare reform efforts may impact our business and the pricing we receive for our services.
In March 2010, healthcare reform legislation known as the “Patient Protection and Affordable Care Act,” also known as the ACA, was passed into law. The ACA makes changes that are expected to significantly impact the pharmaceutical and medical device industries and clinical laboratories. For example, the ACA contains several provisions that seek to limit Medicare spending in the future. One key provision in the ACA is the establishment of “Accountable Care Organizations” (“ACOs”), under which hospitals and physicians are able to share savings that result from improved coordination of healthcare. ACOs continue to develop, and we cannot predict how the continued establishment and implementation of these new business models will impact our business. There is the possibility that value-based payment models, such as ACOs, will drive down the utilization and/or reimbursement rates for our services. We may not be able to gain access into certain ACOs. These changes could have an adverse and material impact on our operations.
Following the 2016 election cycle, there were substantial efforts to repeal all or portions of the ACA. In December 2017, Public Law No. 115-97, which made changes to the tax code and included, among other things, a repeal of the ACA’s penalties for the individual mandate, a provision that required individuals to buy health insurance or pay a fine, became law. On June 17, 2021, the U.S. Supreme Court dismissed a judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. While efforts to repeal all or part of the ACA have subsided, in part due to the results of the 2020 election, we cannot be certain that there will not be further legislative efforts or judicial challenges in the future.
The 2024 presidential election may also significantly alter the current regulatory framework and the health care industry, including any further challenges, extensions or expansions of certain ACA provisions. These changes could have an adverse and material impact on our operations.
Changes in laws, regulations, contracting arrangements with payers, or payer policies, including steps taken by payers to control utilization and reimbursement of healthcare services, may adversely affect coverage or reimbursement for our specialized diagnostic services, which may decrease our revenues and adversely affect our results of operations and financial condition.
Governmental payers, as well as private insurers and private payers, have implemented and will continue to implement measures to control the cost, utilization, and delivery of healthcare services, including clinical laboratory and pathology services. Congress and federal agencies, such as CMS, have, from time to time, implemented changes to laws and regulations governing healthcare service providers, including specialized diagnostic service providers. These changes have adversely affected and may in the future adversely affect coverage for our services. We also believe that healthcare professionals may not use our services if third-party payers do not provide adequate coverage and reimbursement for them. These changes in federal, state, local, and third-party payer regulations or policies may decrease our revenues and adversely affect our results of operations and our financial condition. We will continue to be a non-contracted provider until such time as we enter into contracts with third-party payers with whom we are not currently contracted. Because a portion of our revenues is from third-party payers with whom we are not currently contracted, it is likely that we will be required to make positive or negative adjustments to accounting estimates with respect to contractual allowances in the future, which may adversely affect our results of operations, our credibility with financial analysts and investors, and our stock price.
We face efforts by government payers to reduce utilization as well as reimbursement for laboratory testing services. Changes in governmental reimbursement may result from statutory and regulatory changes, prospective and/or retroactive rate adjustments, administrative rulings, and other policy changes.
From time to time, legislative freezes and updates affect some of our tests that are reimbursed by the Medicare program under the Medicare Physician Fee Schedule (“MPFS”), or the Clinical Laboratory Fee Schedule (“CLFS”). The MPFS is updated on an annual basis. In the past, the MPFS was updated using a prescribed statutory formula (i.e., the sustainable growth rate formula). The Medicare Access and CHIP Reauthorization Act of 2015 (“MACRA”) repealed the previous statutory formula and specified new annual conversion factors for calendar years 2015 and beyond. If the new annual conversion factor results in negative reimbursement in future years, the resulting decrease in payment may adversely affect our revenue, business, operating results, financial condition, and prospects.
29
| NEOGENOMICS, INC. | ||||||||
In addition, recent laws have made changes to Medicare reimbursement for our tests that are reimbursed under the CLFS, many of which have already gone into effect. The Protecting Access to Medicare Act of 2014 (“PAMA”) made significant changes to how Medicare pays for clinical diagnostic laboratory tests under the CLFS. As part of the changes made under PAMA, beginning in 2017, Medicare CLFS reimbursement rates were to be based on the volume-weighted median of the private payer payment rates for these tests. This led to reductions from prior rates, and without further legislative changes, will continue to result in reductions as the Medicare CLFS reimbursement rate converges towards the median private payer rate. Reductions were capped at 10.0 percent per annum from 2017 through 2020, and this cap was set to increase to 15.0 percent for 2020. However, the Coronavirus Aid, Relief, and Economic Security Act (“CARES Act”) and Protecting Medicare and American Farmers from Sequester Cuts Act delayed the implementation of the 15.0 percent rate reduction cap to 2023 and capped reductions at 0.0 percent for 2021 and 2022. The Consolidated Appropriations Act 2023 further delayed the implementation of the 15.0 percent rate reduction cap to 2024 and extended the 15.0 percent rate reduction cap through 2026. The Further Continuing Appropriations and Other Extensions Act of 2024 was passed in 2023 and further delayed the implementation of the 15.0 percent rate reduction cap to 2025 and extended the 15.0 percent rate reduction cap through 2027. When rate reductions begin to take effect again in 2024, this will further reduce Medicare program payments for CLFS tests. It is possible that additional reductions could be enacted in the future.
CMS also adopts regulations and policies, from time to time, revising, limiting, or excluding coverage or reimbursement for certain of the tests that we perform. Likewise, many state governments are under budget pressures and are also considering reductions to their Medicaid fees. Further, Medicare, Medicaid, and other third-party payers audit for overutilization of billed services. Even though all tests performed by us are ordered by our clients who are responsible for establishing the medical necessity for the tests ordered, we may be subject to recoupment of payments, as the recipient of the payments for such tests, in the event that a third-party payer such as CMS determines that the tests failed to meet all applicable criteria for payment. When third-party payers like CMS revise their coverage regulations or policies, our costs generally increase due to the complexity of complying with additional administrative requirements. Furthermore, Medicaid reimbursement and regulations vary by state. Accordingly, we are subject to varying administrative and billing regulations, which also increase the complexity of servicing such programs and our administrative costs. Finally, state budget pressures have encouraged states to consider several courses that may impact our business, such as delaying payments, restricting coverage eligibility, service coverage restrictions and imposing taxes on our services.
In certain jurisdictions, Palmetto GBA administers the Molecular Diagnostic Services Program (“MolDx”) and establishes coverage and reimbursement for certain molecular diagnostic tests, including many of our tests. To obtain Medicare coverage for a molecular diagnostic test (FDA-approved or LDT), laboratories must apply for and obtain a unique test identifier or what is known as a “Z” code. For newly developed tests or for established tests that have not been validated for clinical and analytical validity and clinical utility, laboratories must submit a detailed dossier of clinical data to substantiate that the test meets Medicare’s requirements for coverage. We have received favorable coverage for many of our molecular tests, however, we have also received non-coverage determinations for many newer tests. The field of molecular diagnostics is evolving very rapidly, and clinical studies on many new tests are still underway. We cannot be assured that some of our molecular tests will ever be covered services by Medicare, nor can we determine when the medical literature will meet the standard for coverage that Medicare administrative contractors have set.
In November 2017, CMS initiated a national coverage analysis for the use of NGS diagnostic tests for patients with advanced cancer. The proposed decision memorandum was released and open to a public comment period. On March 16, 2018, CMS issued a final decision memorandum for NGS as a diagnostic laboratory test and determined it to be reasonable and necessary, and covered nationally when performed in a CLIA-certified laboratory, ordered by a treating physician, and all of the following requirements are met: (a) the patient has either recurrent, relapsed, refractory, metastatic, or advanced stages III or IV cancer; (b) the patient has either not been previously tested using the same NGS test for the same primary diagnosis of cancer or has had repeat testing using the same NGS test only when a new primary cancer diagnosis is made by the treating physician; and (c) the patient has decided to seek further cancer treatment (e.g., therapeutic chemotherapy). CMS also determined that the diagnostic laboratory test using NGS must have: FDA approval or clearance as a companion in vitro diagnostic; an FDA approved or cleared indication for use in that patient’s cancer; and results provided to the treating physician for management of the patient using a report template to specify treatment options. On October 29, 2019, CMS issued a proposed decision memorandum open to a public comment period that would expand coverage of NGS test when performed in a CLIA-certified laboratory, ordered by a treating physician, and all of the following requirements are met (a) the patient has ovarian or breast cancer; (b) the patient has clinical indications for germline (inherited) testing; (c) the patient has risk factors for germline (inherited) breast or ovarian cancer; and (d) the patient has not been previously tested using NGS. These CMS changes to reimbursement for NGS testing could directly affect our revenue for this test type.
In recent years, Medicare has encouraged beneficiaries to participate in managed care programs, known as “Medicare Advantage” programs, and has encouraged beneficiaries from the traditional fee-for-service Medicare program to switch to
30
| NEOGENOMICS, INC. | ||||||||
Medicare Advantage programs. This has resulted in rapid growth of health insurance and managed care plans offering Medicare Advantage programs and growth in Medicare beneficiary enrollment in these programs. Also, in recent years, many states have increasingly mandated that Medicaid beneficiaries enroll in managed care arrangements. If these efforts continue to be successful, we may experience a further shift of traditional Medicare and Medicaid fee-for-service beneficiaries to managed care programs. As a result, we would be required to contract with those private managed care programs in order to be reimbursed for services provided to their Medicare and Medicaid members. There can be no assurance that we will be successful in entering into agreements with these managed care programs at rates of payment similar to those we realize from our non-managed care lines of business.
We expect the initiatives such as those described above to continue and, if they do, to reduce reimbursements for clinical laboratory services, to impose more stringent cost controls on clinical laboratory services and to reduce utilization of clinical laboratory services. These efforts, including changes in law or regulations that may occur in the future, may each individually or collectively have a material adverse impact on our business, results of operations, financial condition, and prospects.
Failure to comply with laws and regulations regarding laboratory licensing and operations, including CLIA environmental, health, and safety laws and regulations such as the federal Occupational Safety and Health Administration Act and the Needlestick Safety and Prevention Act, could result in fines and penalties and loss of licensure, and have a material adverse effect upon our business.
We are subject to extensive state and federal regulatory oversight regarding laboratory licensing and operations. Each of our laboratories must satisfy federal requirements under CLIA and maintain the appropriate CLIA Certificate for all testing performed at the lab. Additionally, most states have adopted various laws and regulations setting standards for laboratories performing clinical laboratory testing, and requiring laboratories to obtain and maintain a state laboratory license before the laboratory is authorized to perform testing. These state licensure laws address a host of requirements and often establish permissible and prohibited practices involving digital health, including but not limited to telehealth and telepathology.
Periodic inspections or surveys are performed to determine whether our laboratory locations are compliant with CLIA requirements or with applicable state licensure or certification laws. If we fail to meet any applicable requirements of CLIA or similar state laws, that failure could adversely affect payment for our products and services, prevent their approval entirely, and/or interrupt the commercial sale and/or marketing of any products and services and otherwise cause us to incur significant expense. The sanctions for failure to comply with CLIA, state licensure requirements, or other applicable laws and regulations include the suspension, revocation, or limitation of the right to perform clinical laboratory services or receive compensation for those services, as well as the requirement to enter into a corrective action plan to monitor compliance, and the imposition of civil or criminal penalties or administrative fines. In addition, any new legislation or regulation or the application of existing laws and regulations in ways that we have not anticipated could have a material adverse effect on our business, results of operations, and financial condition.
We are subject to licensing and regulation under federal, state, and local laws and regulations relating to the protection of the environment and human health and safety, including laws and regulations relating to the handling, transportation, and disposal of medical specimens, infectious and hazardous waste, and radioactive materials, as well as regulations relating to the safety and health of laboratory employees. The federal Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for healthcare employers, including clinical laboratories, whose workers may be exposed to blood-borne pathogens such as HIV and the hepatitis B virus. These requirements, among other things, require work practice controls, protective clothing and equipment, training, medical follow-up, vaccinations, and other measures designed to minimize exposure to, and transmission of, blood-borne pathogens. In addition, the Needlestick Safety and Prevention Act requires, among other things, that we include in our safety programs the evaluation and use of engineering controls such as safety needles, if found to be effective at reducing the risk of needlestick injuries in the workplace.
Failure to comply with such federal, state and local laws and regulations could subject us to denial of the right to conduct business, fines, criminal penalties and/or other enforcement actions, any of which could have a material adverse effect on our business. In addition, compliance with future legislation could impose additional requirements for us, which may be costly.
Our net revenue will be diminished if payers do not adequately cover or reimburse our services.
There has been, and will continue to be, significant efforts by both federal and state agencies to reduce costs in government healthcare programs and otherwise implement government control of healthcare costs. In addition, private payers continually seek ways to reduce and control overall healthcare costs, and increasing emphasis on managed care in the United States will continue to put pressure on the pricing of healthcare services. Uncertainty exists as to the coverage and reimbursement status of new applications and services. Third-party payers, including governmental payers such as Medicare and private payers, are scrutinizing new medical products and services and may not cover or may limit coverage and the level of reimbursement for
31
| NEOGENOMICS, INC. | ||||||||
our services. Third-party insurance coverage may not be available to patients for any of our existing tests or for tests we discover and develop, and a substantial portion of the testing for which we bill our hospital and laboratory clients is ultimately paid by third-party payers. Likewise, any pricing pressure exerted by these third-party payers on our clients may, in turn, be exerted by our clients on us. If government and other third-party payers do not provide adequate coverage and reimbursement for our tests, it could adversely affect our operating results, cash flows and/or our financial condition.
Our operations are subject to strict laws prohibiting fraudulent billing and other abuse, and our failure to comply with such laws could result in substantial penalties, including exclusion from participation in Medicare, Medicaid, and other governmental payer programs.
Of particular importance to our operations is ensuring compliance with federal and state laws prohibiting fraudulent billing and the retention of overpayments. In particular, if we fail to comply with federal and state documentation, coding, and billing rules, we could be subject to liability under the federal False Claims Act, including civil penalties, loss of licenses, and exclusion from the Medicare and Medicaid programs. The False Claims Act prohibits individuals and companies from knowingly submitting false claims for payments to, or improperly retaining overpayments from, the government.
If an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus substantial civil penalties for each separate false claim. Further, False Claims Act liability may lead to exclusion from participation in Medicare, Medicaid, and other federal healthcare programs. There are a number of potential bases for liability under the federal False Claims Act. For example, liability arises when an entity knowingly submits, or causes another to submit, a claim for reimbursement to the federal government for a service which was not provided or which did not qualify for reimbursement. Submitting a claim with reckless disregard or deliberate ignorance of its truth or falsity could also result in liability under the False Claims Act. Following enactment of the ACA, knowing retention of overpayments is also considered a false claim and could lead to liability under the False Claims Act.
The False Claims Act’s “whistleblower” or “qui tam” provisions are used with frequency to challenge the reimbursement practices of providers and suppliers. Those provisions allow a private individual to bring an action on behalf of the government alleging that the defendant has submitted false claims for payment to the government. The government must decide whether to intervene in the lawsuit and whether to prosecute the case. If it declines to do so, the individual may pursue the case alone, although the government must be kept apprised of the progress of the lawsuit. Whether or not the federal government intervenes in the case, it will receive the majority of any recovery. The successful qui tam relator who brought the case is entitled to a portion of the proceeds and his or her attorneys’ fees and costs. In addition, various states have enacted laws modeled after the federal False Claims Act, which prohibit submitting false claims for payment to the state, or, in some states, to commercial payers. If we fail to comply with federal and state documentation, coding, and billing rules, we could be subject to liability under analogous state laws as well as criminal liability through a variety of federal and state criminal statutes.
Government investigations of clinical laboratories have been ongoing for a number of years and are expected to continue in the future. Governmental enforcement action or qui tam civil litigation against us may result in material costs and occupy significant management resources, even if we ultimately prevail. In addition, governmental enforcement action may result in substantial fines, penalties or administrative remedies, including exclusion from government reimbursement programs and entry into corporate integrity agreements with governmental agencies, which could entail significant obligations and costs.
When we submit bills for our services to third-party payers, we must follow complex documentation, coding, and billing rules which are based on federal and state laws, rules and regulations, various government publications, and on industry practice. A large number of laboratories have entered into substantial settlements with the federal and state governments for alleged noncompliance under these laws and rules. Private payers have also brought civil actions against laboratories, which have resulted in substantial judgments. Failure to follow these rules could result in potential civil liability under the False Claims Act, under which extensive financial penalties can be imposed. It could further result in criminal liability under various federal and state criminal statutes.
We submit thousands of claims for payment to governmental programs and private payers, and we cannot guarantee that there have not been errors in our claims. While we maintain a robust compliance program that includes consistent, detailed review of our documentation, coding, and billing practices, the rules are frequently vague, complex, and continually changing and we cannot assure that governmental authorities, private insurers, or private whistleblowers will not challenge our practices. Such a challenge could result in a material adverse effect on our business. We therefore could be exposed to potential liability, penalties, or limitations on our operations due to failure to comply with significant government regulation and laboratory operations.
Existing federal laws governing Medicare and Medicaid, as well as other state and federal laws, also regulate certain aspects of the relationship between healthcare providers, including clinical laboratories, and their referral sources, including
32
| NEOGENOMICS, INC. | ||||||||
physicians, hospitals and other laboratories. Some of these laws, including the federal AKS and the federal Stark Law contain extremely broad proscriptions. Violation of these laws can result in criminal or civil penalties, exclusion from participation in the Medicare, Medicaid, and other federal healthcare programs, repayment of reimbursement received related to services tied to any impermissible referrals, or civil monetary penalties, which may be significant, as well as potential False Claims Act liability. Government authorities may determine that our arrangements with physicians and other clients do not comply with the federal AKS, Stark Law, and similar state laws, and may impose civil monetary penalties or exclude us from participation in federal healthcare programs based on our arrangements with physicians and other clients. The Company voluntarily conducted an internal investigation, with the assistance of outside counsel, that focused on the compliance of certain consulting and service agreements with federal healthcare laws and regulations, including those relating to fraud, waste, and abuse. Based on this internal investigation, the Company voluntarily notified the OIG of the Company’s internal investigation in November 2021. The Company’s interactions with regulatory authorities and the Company’s related review of this matter are ongoing. As of December 31, 2023, the Company has accrued a reserve of $11.2 million in other long-term liabilities on the Consolidated Balance Sheets for potential damages and liabilities associated with the federal healthcare program revenue received by the Company in connection with the agreements at issue that were identified during the course of this internal investigation. This reserve reflects management’s best estimate of the minimum probable loss associated with this matter. As a result of the internal investigation and ongoing interactions with regulatory authorities, the Company may accrue additional reserves for any related potential damages and liabilities arising out of this matter. At this time, the Company is unable to predict the duration, scope, result, or related costs associated with any further investigation, including by the OIG, or any other governmental authority, or what penalties or remedial actions they may seek. Accordingly, at this time, the Company is unable to estimate a range of possible loss in excess of the amount reserved. Determinations that the Company’s operations or activities do not, or did not, comply with laws or regulations, however, may result in the imposition of civil or criminal fines, penalties, disgorgement, restitution, equitable relief, exclusion from participation in federal healthcare programs or other losses or conduct restrictions, which could be material to the Company’s financial results or business operations.
The federal Civil Monetary Penalties Law (“Federal CMP Law”) imposes civil monetary penalties and potential exclusion from Medicare and Medicaid programs on any person who offers or transfers remuneration to any patient, who is a Medicare or Medicaid beneficiary, when the person knows or should know that the remuneration is likely to induce the patient to receive medical services from a particular provider. The Federal CMP Law applies, among other things, to many kinds of inducements or benefits provided to patients, including complimentary items or services that are of more than nominal value. Government authorities may determine our operations and provision of services do not comply with the law and its interpretations and impose civil monetary penalties and exclude us from participation in Medicare and Medicaid for past or present practices related to patient incentive, coordination of care and need-based programs.
Tests which are reimbursed by Medicare and other government payers (for example, State Medicaid programs) accounted for approximately 15%, 16% and 18% of our revenues for the years ended December 31, 2023, 2022 and 2021, respectively. The Medicare program imposes extensive and detailed requirements on diagnostic service providers, including, but not limited to, rules that govern how we structure our relationships with physicians, how and when we submit claims for reimbursement, and how we provide specialized diagnostic laboratory services. Further, we are prohibited from contracting with any individuals or entities who have been excluded from participation in Medicare or Medicaid and are listed on the OIG’s List of Excluded Individuals and Entities List (“LEIE”) or in the System for Award Management, which includes the previously independent Government Services Administration’s Excluded Parties List System (“GSA-EPLS”). Contracting with excluded individuals or entities, such as hiring an excluded person or contracting with an excluded vendor, can result in significant penalties.
Our failure to comply with applicable Medicare, Medicaid, and other governmental payer rules could result in our inability to participate in a governmental payer program, an obligation to repay funds already paid to us for services performed, civil monetary penalties, criminal penalties, False Claims Act liability, and/or limitations on the operational function of our laboratory. If we were unable to receive reimbursement under a governmental payer program, a substantial portion of our revenues would be lost, which would adversely affect our results of operations and financial condition.
The failure to comply with fraud and abuse laws, including physician self-referral laws and anti-kickback laws, may subject us to liability, penalties, or limitation of operations.
We are subject to the federal Stark Law, as well as similar state statutes and regulations, which prohibit billing Medicare for certain healthcare services, which are referred to as DHS, rendered as a result of referrals by physicians to DHS entities with which the physicians (or their immediate family members) have a financial relationship unless an exception is met. A “financial relationship” includes both an ownership interest and/or a compensation arrangement with a physician, both direct and indirect, and DHS includes, but is not limited to, laboratory services. The Stark Law prohibits an entity that receives a prohibited DHS referral from seeking payment from Medicare for any DHS services performed as a result of such a referral,
33
| NEOGENOMICS, INC. | ||||||||
unless an arrangement is carefully structured to satisfy every requirement of a regulatory exception. The Stark Law is a strict liability statute, and thus any technical violation requires repayment of all “tainted” referrals, regardless of the intent, unless an exception applies. Penalties for violating the Stark Law may include the denial of payment to an entity for the impermissible provision of DHS, the requirement to refund any amounts collected in violation of the Stark Law, and substantial civil monetary penalties for each circumvention arrangement or scheme. Other implications of a Stark Law violation may include exclusion from Medicare and Medicaid programs, and potential False Claims Act liability, including via “qui tam” action.
Further, many states have promulgated self-referral laws and regulations similar to the federal Stark Law, and these vary significantly based on the state. In addition to services reimbursed by Medicaid or government payers, these state laws and regulations can encompass services reimbursed by private payers and self-pay patients as well. Penalties for violating state self-referral laws and regulations vary based on the state, but often include civil penalties, exclusion from Medicaid, and loss of licenses.
Our financial arrangements with physicians are governed by the federal Stark Law, and we rely on certain exceptions to the Stark Law with respect to such relationships. If we are found by the government to be in violation of the Stark Law, we could be subject to significant penalties, including fines as specified above, exclusion from participation in government and private payer programs and requirements to refund amounts previously received from government. Further, as our operations expand into new states and jurisdictions, we must continually evaluate whether our relationships with physicians comply with such new jurisdiction’s laws. This may require structural and organizational modifications to our relationships with physicians, which could adversely affect our results of operations and financial condition.
We are subject to the federal AKS, which is a criminal felony statute that prohibits the knowing and willful offer, payment, solicitation, or receipt of any form of remuneration in return for referring, ordering, leasing, purchasing, or arranging for or recommending the ordering, purchasing, or leasing of items or services payable by Medicare, Medicaid, or any other federally funded healthcare program. Remuneration has been broadly interpreted to include anything of value, in cash or in kind, and thus can implicate financial relationships involving payments not commensurate with fair market value, such as in the form of office space, equipment leases, professional or technical services, or anything else of value.
The AKS is an “intent-based” statute, meaning that a violation occurs when one or both parties intend the remuneration to be in exchange for or to induce referrals. In 2010, the ACA, amended the intent requirement of the AKS. A person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it. In addition, the ACA provides that a claim submitted for reimbursement for items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the federal False Claims Act.
There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions; however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny. Violations of the AKS may result in substantial civil or criminal penalties, including criminal fines, imprisonment, civil penalties under the Federal CMP Law, civil penalties and damages under the federal False Claims Act and exclusion from participation in the Medicare and Medicaid programs. If we face these penalties or exclusion from participation in Medicare and Medicaid, it could significantly reduce our revenues and could have a material adverse effect on our business. Further, non-compliant activities and unlawful conduct by sales and marketing personnel could give rise to significant risks under the AKS. We require extensive, comprehensive training of all sales and marketing personnel, but cannot guarantee that every staff member will comply with the training. Thus, we could face liability under the AKS for non-compliance by individuals engaged in prohibited sales and marketing activities.
Further, most states have adopted similar anti-kickback laws prohibiting the offer, payment, solicitation, or receipt of remuneration in exchange for referrals, and typically impose criminal and civil penalties as well as loss of licenses. Some of these state laws apply to items and services paid for by private payers as well as by government payers. In addition, many states have adopted laws prohibiting the splitting or sharing of fees between physicians and non-physicians, as well as between treating physicians and referral sources. If we are found to be in violation of the AKS or a similar state anti-kickback law, we could be subject to significant penalties, including fines, exclusion from participation in government and private payer programs, or obligations to refund amounts previously received from government payers. We also could be required to restructure or terminate our contractual and other arrangements with physicians, which could result in a loss of revenue and have a material adverse effect on our business.
Some states have also adopted laws prohibiting the corporate practice of medicine, or prohibiting business corporations from employing physicians or engaging in activities considered to be the “practice of medicine.” In these states, we rely on service agreements with physicians and/or professional associations owned by physicians, to perform needed professional pathology services. We cannot be certain that a physician or physician’s professional organization will not seek to terminate an
34
| NEOGENOMICS, INC. | ||||||||
agreement with us on any basis, nor can we be certain that governmental authorities in those states will not seek termination of these arrangements on the basis of state laws prohibiting the corporate practice of medicine.
Failure to comply with federal, state and international laws related to privacy and security could result in fines, penalties, and damage to the Company’s reputation with customers and could have a material adverse effect upon the Company’s business.
In the U.S., HIPAA, as expanded through the HITECH Act and as implemented through the HIPAA Rules, and similar state laws contain provisions that require the electronic exchange of health information, such as claims submission and receipt of remittances, using standard transactions and code sets, which we refer to as “Standards,” and regulate the use and disclosure of patient records and other PHI. These provisions, which address security and confidentiality of patient information as well as the administrative aspects of claims handling, have very broad applicability and govern many healthcare providers, including physicians and clinical laboratories. Failure to comply with the Standards, the HIPAA Rules, and applicable state privacy and security laws, could result in material adverse effects on our business, results of operations, and our financial condition and could subject us to liability.
The HIPAA Rules establish comprehensive federal standards with respect to the uses and disclosures of PHI by certain entities including health plans and healthcare providers, and set standards to protect the confidentiality, integrity, and availability of electronic medical records. The regulations establish a complex regulatory framework governing the use and disclosure of PHI, including, for example, the circumstances under which uses and disclosures of PHI are permitted or required without a specific authorization by the patient; a patient’s right to access, amend, and receive an accounting of certain disclosures of PHI; the content of notices of privacy practices describing how PHI is used and disclosed and individuals’ rights with respect to their PHI; and implementation of administrative, technical, and physical safeguards to protect privacy and security of PHI. The federal privacy regulations restrict our ability to use or disclose certain individually identifiable patient health information, without patient authorization, for purposes other than payment, treatment, or healthcare operations, as defined by HIPAA, except for disclosures for various public policy purposes and other permitted purposes outlined in the HIPAA Rules. The HIPAA Rules do not supersede state laws that may be more stringent; therefore, we are required to comply with both federal privacy and security regulations and varying state privacy and security laws and regulations.
The HIPAA Rules also require healthcare providers like us to notify affected individuals, the Secretary of the U.S. Department of Health and Human Services, and in some cases, the media, when PHI has been “breached,” as defined by HIPAA. Many states have similar breach notification laws. In the event of a breach, we could incur substantial operational and financial costs related to mitigation and remediation, including preparation and delivery of notices to affected individuals. Additionally, HIPAA and its implementing regulations provide for significant civil fines, criminal penalties, and other sanctions for failure to comply with the privacy, security, and breach notification rules, including for wrongful or impermissible use or disclosure of PHI. Although the HIPAA statute and regulations do not expressly provide for a private right of action for damages, we could incur damages under state laws to private parties for the wrongful or impermissible use or disclosure of confidential health information or other private personal information. Additionally, HIPAA allows state Attorneys General to bring an action against a covered entity, such as us, for a violation of HIPAA. We insure some of our risk with respect to HIPAA security breaches, but operational costs and penalties associated with HIPAA breaches easily could exceed our insured limits.
HIPAA imposes additional requirements, restrictions, and penalties on covered entities and their business associates to, among other things, deter breaches of security. As a result, in addition to the aforementioned reporting requirements, covered entities and their business associates may be required to take preventative and remedial actions, as well as face stringent sanctions for a breach. Our electronic health records system is periodically modified to meet applicable security standards. Despite our implementation of various security measures, our infrastructure may be vulnerable to computer viruses, break-ins, and other disruptive problems inadvertently introduced by authorized users such as employees and clients, or purposefully targeted by hackers and other cybercriminals which could lead to interruption, delays, or cessation in service to our clients. Further, such incidents, whether electronic or physical, could jeopardize the security of confidential information, including PHI and other sensitive information stored in our computer systems related to clients, patients, and other parties connected through us, which may deter potential clients and give rise to uncertain liability to parties whose security or privacy has been infringed. A significant security breach could result in fines, loss of clients, damage to our reputation, direct damages, costs of repair and detection, costs to remedy the breach, government penalties, and other expenses. We insure some of our risk with respect to security breaches but the occurrence of any of the foregoing events could have a material adverse effect on our business, results of operations, and our financial condition.
In the United States, in addition to the HIPAA Rules described above, the Company is subject to additional federal and state laws regarding the handling and disclosure of patient records and patient health information. Effective April 5, 2021, HHS
35
| NEOGENOMICS, INC. | ||||||||
published a final rule implementing the information blocking provisions (“Information Blocking Rules”) of the 21st Century Cures Act. The Information Blocking Rules prohibit covered actors, including healthcare providers, from engaging in activity that is likely to interfere with the access, exchange, or use of EHI unless such activity falls into one of eight exceptions. The Information Blocking Rules provide for civil monetary penalties for noncompliance by healthcare IT vendors and, separately, “appropriate disincentives” for noncompliance by healthcare providers.
The HIPAA Rules do not supersede state laws that may be more stringent; therefore, we are required to comply with both federal privacy and security regulations as well as varying state privacy and security laws and regulations. These laws vary widely. For example, many states have implemented genetic testing and privacy laws imposing specific patient consent requirements and limiting the disclosure of genetic test results. Penalties for violation include sanctions against a laboratory’s licensure as well as civil or criminal penalties. Additionally, private individuals may have a right of action against the Company for violations of a state’s privacy laws.
Numerous other federal, state, and international laws govern the collection, use, and disclosure of personal information and may complicate our compliance efforts. Failure to comply with these laws can result in the imposition of significant fines and impact our ability to process certain personal data. For example, in the U.S., the CCPA affords California residents expanded privacy rights and protections and provides for civil penalties for violations and a private right of action related to certain data security breaches. These protections have been expanded by the CPRA, which became operational in most key respects on January 1, 2023. Similar laws have been proposed or passed at the U.S. federal and state level, including the Virginia Consumer Data Protection Act, which took effect on January 1, 2023, the Colorado Consumer Protection Act, which took effect on July 1, 2023, the Connecticut Data Privacy Act, which took effect on July 1, 2023, and the Utah Consumer Privacy Act, which took effect on December 31, 2023. A number of other states have enacted laws related to the privacy and security of consumer health information and personal data which will become effective within the next two years, including Delaware, Florida, Indiana, Iowa, Montana, Nevada, Oregon, Tennessee, Texas, and Washington, and more states have proposed legislation under consideration. The legislative and regulatory landscape for privacy and data protection continues to evolve, and there has been an increasing amount of focus on privacy and data protection issues with the potential to affect our business, including laws in all 50 states requiring security breach notification in some circumstances. These and other laws could increase regulatory compliance risk, create liability for us or increase our cost of doing business.
Outside of the U.S., the European Union's data privacy law, the GDPR, for example, imposes penalties of up to 4.0% of annual global revenue. The GDPR imposes a number of strict obligations and restrictions on the ability to process (which includes collection, analysis, and transfer of) personal data, including health data from performance of clinical tests, clinical trials and adverse event reporting. The GDPR also includes requirements relating to establishing a legal basis for processing personal data, the information provided to the individuals prior to processing their personal data or personal health data, notification of data processing obligations to the national data protection authorities, standards for binding vendors that process personal data, and the security and confidentiality of the personal data. Further, the GDPR prohibits the transfer of personal data to countries outside of the EU that are not considered by the European Commission to provide an adequate level of data protection, including to the United States, except if the data controller meets very specific requirements. In July 2020, the Court of Justice of the European Union (CJEU) invalidated the E.U.-U.S. Privacy Shield Framework, under which personal data could be transferred from the EEA to U.S. entities that had self-certified under the Privacy Shield scheme. This framework has been replaced by the E.U.-U.S. Data Privacy Framework for which the European Commission adopted an adequacy decision in July 2023. It is likely there will be legal challenges to this framework in the future, which could draw into question the legitimacy of other cross-border transfer mechanisms, including the standard contractual clauses which remain a commonly used mechanism used to transfer personal data from the EEA to the U.S. and other jurisdictions.
These recent developments may require us to review and amend the legal mechanisms by which we make and/ or receive personal data transfers to/ in the United States. As supervisory authorities issue further guidance on personal data export mechanisms, including circumstances where the standard contractual clauses cannot be used, and/or start taking enforcement action, we could suffer additional costs, complaints and/or regulatory investigations or fines, and/or if we are otherwise unable to transfer personal data between and among countries and regions in which we operate, it could affect the manner in which we provide our services, the geographical location or segregation of our relevant systems and operations, and could adversely affect our financial results.
General Risk Factors
We are dependent on key personnel and need to hire additional qualified personnel in order for our business to succeed.
Our performance is substantially dependent on the performance of our senior management and key scientific and technical personnel. In particular, our success depends substantially on the continued efforts of our senior management team. The loss
36
| NEOGENOMICS, INC. | ||||||||
of the services of any of our executive officers, our medical staff, our laboratory directors or other key employees could have a material adverse effect on our business, results of operations, and our financial condition. Our future success also depends on our continuing ability to attract and retain highly qualified managerial, scientific, and technical personnel as we continue to grow. Competition for such personnel is intense among the laboratory testing industry and we may not be able to retain our key managerial and technical employees or may not be able to attract and retain additional highly qualified managerial and technical personnel in the future. The inability to attract and retain the necessary managerial and technical personnel could have a material adverse effect upon our business, results of operations, and financial condition.
Additionally, our ability to retain existing clients for our specialized diagnostic services and attract new clients is dependent upon retaining existing sales representatives and hiring and training new sales representatives, which are expensive and time-consuming processes. Our growth depends, in particular, on attracting, retaining and motivating highly-trained sales personnel with the necessary scientific background and ability to understand our systems at a technical level to effectively identify and sell to potential new customers.We face intense competition for qualified sales personnel and our inability to hire or retain an adequate number of sales representatives could limit our ability to maintain or expand our business and increase sales. Even if we are able to increase our sales force, our new sales personnel may not commit the necessary resources or provide sufficient high quality service and attention to effectively market and sell our services. If we are unable to maintain and expand our marketing and sales networks, or if our sales personnel do not perform to our standards, we may be unable to maintain or grow our existing business and our results of operations and financial condition will likely suffer accordingly. If a sales representative ceases employment, such termination could result in the loss of client goodwill based on the impairment of relationships developed between the sales representative and the healthcare professionals for whom the sales representative was responsible. This is particularly a risk if the representative goes to work for a competitor, as the healthcare professionals that are our clients may choose to use a competitor’s services based on their relationship with our former sales representative.
We may not be able to implement our business strategy, which could impair our ability to continue operations.
Implementation of our business strategies will depend in large part on our ability to (i) attract and maintain a significant number of clients; (ii) effectively provide acceptable products and services to our clients; (iii) develop and license new products and technologies; (iv) obtain adequate financing on favorable terms to fund our business strategies; (v) maintain appropriate internal procedures, policies, and systems; (vi) hire, train, and retain skilled employees and management; (vii) continue to operate despite competition in the medical laboratory industry; (viii) be paid reasonable fees by government payers that will adequately cover our costs; (ix) establish, develop, and maintain our name recognition; and (x) establish and maintain beneficial relationships with third-party insurance providers and other third-party payers. Our inability to obtain or maintain any or all these factors could impair our ability to implement our business strategies successfully, which could have material adverse effects on our results of operations and financial condition.
We may be unable to realize estimated benefits from our cost reduction and restructuring efforts and our profitability may be hurt or our business might otherwise be adversely affected.
We engaged in restructuring activities beginning in 2022 and these types of cost reduction and restructuring activities are ongoing and complex. If we do not successfully manage our current restructuring activities, or any other restructuring activities that we may take in the future, any expected efficiencies and benefits might be delayed or not realized, and our operations and business could be disrupted. Restructuring presents potential risks of events occurring that could adversely affect us, including: actual or perceived disruption of service to customers; the failure to preserve supplier relationships and distribution, sales and other important relationships and to resolve conflicts that may arise; diversion of management attention from ongoing business activities; and the failure to maintain employee morale and retain key employees. In addition, the costs associated with implementing restructuring activities might exceed expectations, which could result in additional future charges. Because of these and other factors, we cannot predict whether we will realize the purpose and anticipated benefits of these measures and, if we do not, our business and results of operations may be adversely affected.
If we are unable to successfully integrate future acquisitions with our business, the anticipated benefits of such transaction may not be realized and our business, financial conditions, results of operations and cash flows may be adversely affected.
Acquisitions require us to devote significant management attention and resources to integrating the acquired company’s business practices and operations with our own. Potential difficulties we may encounter as part of the integration process, include the following:
•the potential inability to successfully combine the acquired company’s business with our business in a manner that permits us to achieve the cost synergies expected to be achieved when expected, or at all, and other benefits anticipated to result from such transaction;
37
| NEOGENOMICS, INC. | ||||||||
•challenges optimizing the customer information and technology of the two companies, including the goal of consolidating to one laboratory information system and one billing system;
•challenges effectuating any diversification strategy, including challenges achieving revenue growth from sales of each company’s products and services to the customers of the other company;
•difficulties offering products and services across our expanded portfolio;
•the need to revisit assumptions about reserves, revenues, capital expenditures, and operating costs, including expected synergies;
•challenges faced by a potential diversion of the attention of our management as a result of the integration, which in turn could adversely affect our ability to maintain relationships with customers, employees and other constituencies or our ability to achieve the anticipated benefits of such transaction;
•the potential loss of key employees, customers, managed care contracts, or strategic partners, or the ability to attract or retain key management and other key personnel, which could have an adverse effect on our ability to integrate and operate the acquired business;
•complexities associated with managing the combined businesses, including difficulty addressing possible differences in corporate cultures and management philosophies and the challenge of integrating complex systems, technology, networks, and other assets of each of the companies in a seamless manner that minimizes any adverse impact on customers, suppliers, employees, and other constituencies;
•costs and challenges related to the integration of the acquired company’s internal controls over financial reporting with ours; and
•potential unknown liabilities and unforeseen increased expenses.
We cannot be assured that all of the goals and anticipated benefits of an acquisition will be achievable, particularly as achievement of the benefits is in many important respects subject to factors that we do not control. These factors would include the reactions of third parties with whom we enter into contracts and do business and the reactions of investors and analysts.
If we cannot successfully integrate our business with any future business we may acquire, we may fail to realize the expected benefits of such transaction, including the anticipated cost synergies, and our business, financial condition, results of operations and cash flows may be materially and adversely affected. We could also encounter additional transaction and integration costs or be subject to other factors that affect preliminary estimates.
In the future, we may enter into transactions to acquire other businesses, products, services or technologies, which may ultimately be unsuccessful. If we do identify suitable candidates, we may not be able to make such acquisitions on favorable terms or at all. Any acquisitions we make may not strengthen our competitive position, and these transactions may be viewed negatively by investors, healthcare providers, patients and others. In addition to the risks outlined above, we may decide to incur debt in connection with an acquisition or issue our common stock or other securities to the stockholders of the acquired company, which would reduce the percentage ownership of our existing stockholders. We cannot predict the number, timing or size of future acquisitions or the effect that any such transactions might have on our operating results.
If goodwill and intangible assets that we recorded in connection with our acquisitions become impaired, we may have to take significant charges against earnings.
In connection with the accounting for our completed acquisitions, we recorded a significant amount of goodwill and intangible assets. Goodwill and indefinite-lived intangible assets are evaluated for impairment annually, or more frequently if conditions warrant, by comparing the carrying value of a reporting unit to its estimated fair value. Intangible assets with definite lives are reviewed for impairment when events or circumstances indicate that their carrying value may not be recoverable. Declines in operating results, sustained market declines and other factors that impact the fair values of our reporting units could result in an impairment of goodwill or intangible assets and a charge against earnings, which could materially adversely affect our results of operations or financial condition in future periods.
We may incur greater costs than anticipated in connection with implementing our business strategy, which could result in sustained losses.
We use reasonable efforts to assess and predict the expenses necessary to pursue our business strategies. However, implementing our business strategy may require more employees, capital equipment, supplies, or other expenditure items than management has predicted, particularly as we continue to assess any further needs resulting from the growth of our Advanced Diagnostics segment. Similarly, the cost of compensating additional management, employees, and consultants or other operating costs may be more than we estimate, which could result in ongoing and sustained losses.
38
| NEOGENOMICS, INC. | ||||||||
We may face fluctuations in our results of operations and we are subject to seasonality in our business which could negatively affect our business operations.
Management expects that our results of operations may fluctuate significantly in the future as a result of a variety of factors, including, but not limited to: (i) the continued rate of growth, usage, and acceptance of our products and services; (ii) demand for our products and services; (iii) the introduction and acceptance of new or enhanced products or services by us or by competitors; (iv) our ability to anticipate and effectively adapt to developing markets and to rapidly changing technologies; (v) our ability to attract, retain, and motivate qualified personnel; (vi) the initiation, renewal, or expiration of significant contracts with any major clients; (vii) pricing changes by us, our suppliers, or our competitors; (viii) seasonality; and (ix) general economic conditions and other factors. Accordingly, future sales and operating results are difficult to forecast. Our expenses are based in part on our expectations as to future revenues and to a significant extent are relatively fixed, at least in the short-term. We may not be able to adjust spending in a timely manner to compensate for any unexpected revenue shortfall. Accordingly, any significant shortfall in relation to our expectations would likely have an immediate adverse impact on our business, results of operations, and financial condition. In addition, we may determine from time to time to make certain pricing or marketing decisions or acquisitions that could have a short-term material adverse effect on our business, results of operations, and financial condition and may not result in the long-term benefits intended. Furthermore, in Florida, historically our largest referral market for laboratory testing services, a meaningful percentage of the population returns to their homes in the Northern United States to avoid the hot summer months. This, combined with our clients’ usual summer vacation schedules typically results in seasonality in our business. Because of all of the foregoing factors, our operating results in future periods could be less than the expectations of investors. See Part I, Item 1, “Business—Seasonality” in this Annual Report on Form 10-K for further discussion of the seasonality of our business.
The steps we have taken to protect our intellectual property and proprietary rights may not be adequate, which could result in infringement or misappropriation by third parties.
We regard our copyrights, trademarks, trade secrets, and similar intellectual property as critical to our success, and we rely upon trademark law, copyright law, trade secret protection, and confidentiality and/or license agreements with our employees, clients, partners, and others to protect our proprietary rights. The steps taken by us to protect our proprietary rights may not be adequate or third parties may infringe or misappropriate our copyrights, trademarks, trade secrets, and similar proprietary rights. In addition, other parties may assert infringement claims against us.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 1C. CYBERSECURITY
Our information security program is managed by the President of Enterprise Operations, whose team is responsible for leading enterprise-wide cybersecurity strategy, policy, standards, architecture, and processes. The President of Enterprise Operations provides periodic reports to the Audit Committee and the full Board of Directors (the “Board”), as well as our Chief Executive Officer and other members of our senior management as appropriate. These reports include updates on the Company’s cyber risks and threats, the status of key initiatives to strengthen the information security profile of our systems, cybersecurity incident response readiness, assessments of the information security program, and the emerging threat landscape. Our program is regularly evaluated by internal and external experts with the results of those reviews reported to senior management and the Board. We also actively engage with key vendors, industry participants, and intelligence and law enforcement communities as part of our continuing efforts to evaluate and enhance the effectiveness of our information security policies and procedures.
ITEM 2. PROPERTIES
We operate an international network of laboratories. Our leases expire at various dates through 2041. We believe that these locations are sufficient to meet our needs at existing volume levels and, if needed, additional space will be available at a reasonable cost.
39
| NEOGENOMICS, INC. | ||||||||
We maintain laboratories at all of our facilities, as well as administrative offices at four of our locations. The following table summarizes our facilities by location and approximate square footage:
| Location | Square Footage | |||||||
| Durham, North Carolina | 187,700 | |||||||
| Fort Myers, Florida | 150,000 | |||||||
| Aliso Viejo, California | 112,700 | |||||||
| Carlsbad, California | 28,600 | |||||||
| Houston, Texas | 28,100 | |||||||
| San Diego, California | 25,400 | |||||||
| Cambridge, United Kingdom | 12,500 | |||||||
| Nashville, Tennessee | 7,800 | |||||||
| Tampa, Florida | 5,600 | |||||||
| Phoenix, Arizona | 4,700 | |||||||
| Atlanta, Georgia | 3,800 | |||||||
| Fresno, California | 2,600 | |||||||
| Chicago, Illinois | 2,200 | |||||||
Our Nashville, Tennessee; Tampa, Florida; Atlanta, Georgia; and Phoenix, Arizona locations support our Clinical Services segment exclusively. All other locations serve both segments of the business. For further financial information about our segments, please refer to Note 17. Segment Information, in the notes to our Consolidated Financial Statements.
ITEM 3. LEGAL PROCEEDINGS
From time to time the Company is engaged in legal proceedings, including proceedings that arise in the ordinary course of business. For further information on legal proceedings, please refer to Note 15. Commitments and Contingencies, in the notes to our Consolidated Financial Statements.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is listed on The Nasdaq Stock Market LLC under the symbol “NEO.”
Holders of Common Stock
As of February 13, 2024, there were 645 stockholders of record of our common stock. The number of record holders does not include beneficial owners of common stock whose shares are held in the names of banks, brokers, nominees or other fiduciaries.
Dividends
We have never declared or paid cash dividends on our common stock. We intend to retain all future earnings to finance operations and future growth and, therefore, we do not anticipate paying any cash dividends in the foreseeable future. Our financing arrangements contain certain restrictions on our ability to pay dividends on our common stock.
Recent Sales of Unregistered Securities
None for the year ended December 31, 2023 that have not been previously included in a Current Report on Form 8-K.
40
| NEOGENOMICS, INC. | ||||||||
Issuer Purchases of Equity Securities
The following table sets forth information concerning our purchases of common stock for the periods indicated:
| Period of Repurchase | Total Number of Shares Purchased (1) |
Average Price Paid per Share | Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs | Maximum Number (or Approximate Dollar Value) of Shares that May Yet Be Purchased Under the Plans or Programs | |||||||||||||||||||
October 1, 2023 - October 31, 2023 |
425 | $ | 12.31 | — | — | ||||||||||||||||||
November 1, 2023 - November 30, 2023 |
2,365 | $ | 14.03 | — | — | ||||||||||||||||||
December 1, 2023 - December 31, 2023 |
14,819 | $ | 18.01 | — | — | ||||||||||||||||||
| Total | 17,609 | — | — | ||||||||||||||||||||
(1) The Company’s 2023 Equity Incentive Plan, adopted on May 25, 2023, allows participants to surrender vesting shares having a fair market value equal to the required withholding tax related to the vesting of restricted stock. Pursuant to a share withholding election made by participants in connection with the vesting of such awards, all of which were outside of a publicly-announced repurchase plan, we acquired from such participants the shares noted in the table above to satisfy tax withholding obligations related to the vesting of their restricted stock. The average prices listed in the above table are averages of the fair market prices at which we valued shares withheld for purposes of calculating the number of shares to be withheld.
Comparison of Cumulative Five Year Total Return
We have presented below the cumulative total return to our stockholders of $100 during the period from December 31, 2018, through December 31, 2023, in comparison to the cumulative return on the S&P 500 Index, the Nasdaq Biotechnology Index (^NBI) and a customized peer group of five publicly traded companies during that same period. The peer group is made up of Invitae Corporation, Exact Sciences Corporation, Laboratory Corporation of America Holdings, Natera, Inc., and Quest Diagnostics, Inc. Several of our closest competitors are part of large pharmaceutical or other multi-national firms, or are privately held and, as such, we are unable to obtain financial information for them.
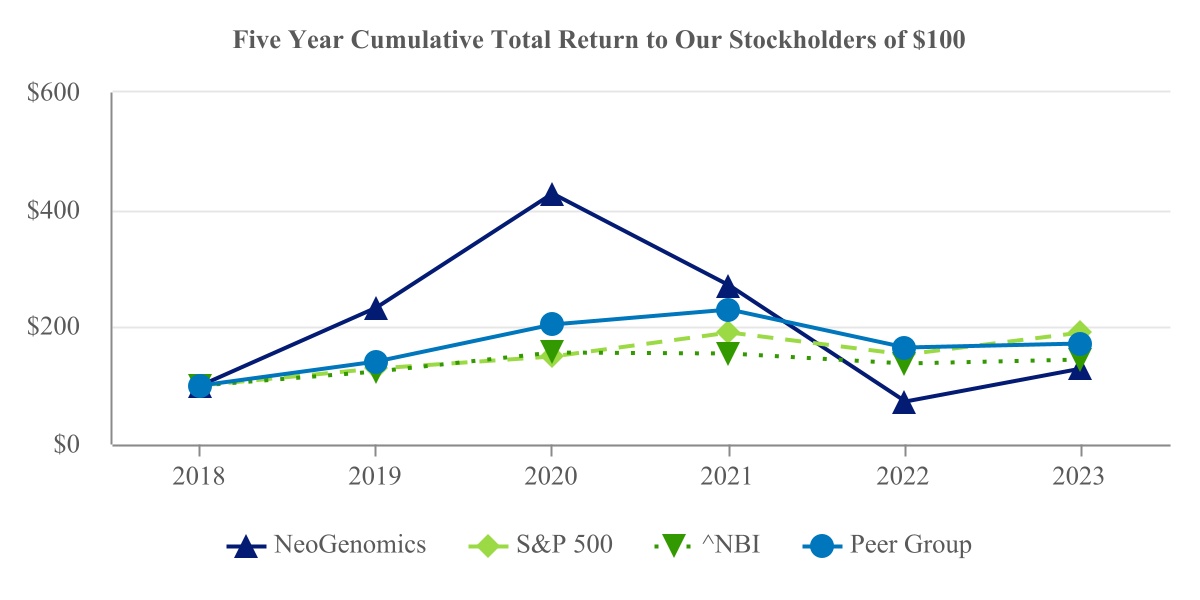
The results assume that $100 (with reinvestment of all dividends) was invested in our common stock, the index, and in the peer group and its relative performance tracked through December 31, 2023. The comparisons are based on historical data and are not indicative of, nor intended to forecast, the future performance of our common stock. The performance graph set forth above shall not be deemed incorporated by reference into any filing by us under the Securities Act of 1933, as amended (the “Securities Act”) or the Exchange Act except to the extent that we specifically incorporate such information by reference therein.
ITEM 6. [RESERVED]
41
| NEOGENOMICS, INC. | ||||||||
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Introduction
The following discussion and analysis should be read in conjunction with the Consolidated Financial Statements and the Notes thereto included in this Annual Report on Form 10-K. The information contained below includes statements of management’s beliefs, expectations, goals and plans that, if not historical, are forward-looking statements subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. For a discussion on forward-looking statements, see the information set forth in the introductory note to this Annual Report under the caption “Forward Looking Statements,” which information is incorporated herein by reference. For discussion and analysis pertaining to 2022 overview and highlights as compared to 2021, please refer to the Company’s Annual Report on Form 10-K, filed with the Securities and Exchange Commission (“SEC”) on February 24, 2023.
Our Company
NeoGenomics, Inc., a Nevada corporation (the “Parent,” “Company,” or “NeoGenomics”), and its subsidiaries provide a wide range of oncology diagnostic testing and consultative services which includes technical laboratory services and professional interpretation of laboratory test results by licensed physicians who specialize in pathology and oncology. The Company operates a network of cancer-focused testing laboratories in the United States and the United Kingdom.
2023 Overview and Highlights
•We increased consolidated revenue by 16.1% compared to 2022, including increases in Clinical Services revenue of 18.4% and in Advanced Diagnostics Services revenue of 5.5%;
•Net cash used in operations improved $64.0 million compared to 2022;
•We increased Adjusted EBITDA $51.5 million to positive $3.5 million compared to in 2022; and
•We improved gross margin by 448 basis points while also improving turnaround time.
Company Outlook
Advances in science and technology are driving a proliferation of oncology therapies and associated diagnostic tests. These diagnostic tools and therapies are increasing survival and enhancing quality of life for cancer patients. As a leading global oncology diagnostics company serving biopharmaceutical companies as well as practicing oncologists and pathologists, NeoGenomics facilitates the adoption of these advanced oncology diagnostic tools beyond the academic environment into the community setting. We are continuously enhancing and expanding our test menu to ensure that providers and patients have access to leading edge solutions such as advanced molecular testing and state-of-the art digital pathology. Moreover, our team of MDs and PhDs, along with our highly-trained oncology-focused sales team, provides ongoing education to our clients to ensure that they remain abreast of cutting-edge developments in oncology.
We are a leading provider of oncology-diagnostic services to biopharma companies. We will continue to work with these clients across the drug development continuum—from research and development through clinical trials testing—to commercialization of companion diagnostic tests. We expect to continue to grow our Advanced Diagnostics business through (i) expansion of our test offerings (including leading edge NGS tools such as WES, WGS, and others), and (ii) our unique capabilities for developing and commercializing companion diagnostic tests.
We are continuing to develop and broaden our informatics and data-related tools to leverage our strategic market position and oncology expertise to help our stakeholders solve real-world problems such as identifying patients for clinical trials or providing clinical decision support tools for physicians and providers. We are committed to connecting patients with life-altering therapies and trials. In carrying out these commitments, NeoGenomics aims to provide transparency and choice to patients regarding the handling and use of their data through our Notice of Privacy Practices, and has invested in leading technologies to help ensure the data we maintain is secured at all times.
We believe lower cost and increased value of testing is extremely important to the healthcare industry and creates a competitive advantage. We will invest in information technology, automation and best practices to continually improve our processes and drive down the cost of testing. We will continue to expand our test menu and expect to remain at the forefront of the ongoing revolution in cancer related genetic and molecular testing to achieve our vision of becoming one of the world’s leading cancer testing and information companies.
We continue to develop our company-wide focus, which includes the following four critical success factors for 2024:
42
| NEOGENOMICS, INC. | ||||||||
Profitably Grow Core Business
•Grow volume and NGS mix;
•Drive market penetration;
•Win on oncology; and
•Improve revenue cycle management.
Accelerate Advanced Diagnostics
•Execute Neo Comprehensive 2.0 launch;
•Execute liquid biopsy CGP launch; and
•Improve gross margin.
Drive Value Creation
•Increase productivity and efficiency;
•Improve gross margin;
•Implement LIMS Phase 1; and
•Prioritize quality system enhancements.
Enhance Our People and Culture
•Enhance teammate development and engagement; and
•Grow a customer-oriented and growth mindset.
These critical success factors have been communicated throughout our Company. We have structured departmental goals around these factors and have created employee incentive plans in which every employee will have a meaningful incentive for our success.
Regulatory Environment
The FDA is currently considering changes that may include increased regulation of LDTs by the FDA. In October 2014 the FDA announced its proposed framework and timetable and indicated it would move toward greater oversight of LDTs. The FDA has not finalized the framework they announced in 2014. In 2017 the FDA shifted its approach to oversight of LDTs, indicating that they would work with Congress and stakeholders on a new legislative framework and pathway for all diagnostic testing. In 2018 the FDA began limited enforcement activities on a subset of LDTs known as pharmacogenetic testing (“PGx”). NeoGenomics is a member of the American Clinical Laboratory Association (“ACLA”), which has been in active discussions with the FDA and Congress regarding the FDA oversight of LDT’s. However, recent agency announcements made in the context of the COVID-19 public health emergency have produced a shifting policy landscape and further uncertainty regarding the FDA’s role in regulating LDTs: in August 2020, HHS announced that the FDA would not require premarket review of LDTs absent notice-and-comment rulemaking, but in November 2021, HHS issued a statement withdrawing that prior announcement, indicating a return to FDA’s longstanding approach to the regulation and enforcement discretion toward LDTs. The most recent such proposal, the VALID Act, was introduced in both the House and Senate on June 24, 2021. The VALID Act was expected to be included in the Omnibus bill signed at the end of 2022, but ultimately was not included and that, as such, it remains unclear whether the VALID Act will be passed 2023 or whether FDA will proceed through rulemaking. At this time, we cannot predict what the current administration's impact will be on the oversight and regulation of LDTs or if there will be any changes to current rules and regulations.
We closely monitor changes in legislation and take specific actions to identify and estimate the impact of changes in legislation whenever possible as regulatory changes can affect reimbursement for clinical laboratory services. We do not anticipate significant changes to our clinical revenue in 2024 resulting from known changes in legislation or rulemaking.
Reportable Segments
We report our activities in two reportable segments—the Clinical Services Segment and the Advanced Diagnostics Segment. We have presented the financial information reviewed by the Chief Operating Decision Maker including revenues, cost of revenue, and gross margin for each of our reportable segments. Assets are not presented at the segment level as that information is not used by the CODM.
Clinical Services
The clinical cancer testing services we offer to community-based pathologists and oncologists are designed to be a natural extension of, and complementary to, the services that they perform within their own practices. We believe our relationship as
43
| NEOGENOMICS, INC. | ||||||||
a non-competitive partner to community-based pathology practices, hospital pathology labs, reference labs, and academic centers can empower them to expand their breadth of testing to provide a menu of services that could match or exceed the level of service found in any center of excellence around the world. Community-based pathology practices and hospital pathology labs may order certain testing services on a technical component only (“TC” or “tech-only”) basis, which allows them to participate in the diagnostic process by performing the professional component (“PC”) interpretation services without having to hire laboratory technologists or purchase the sophisticated equipment needed to perform the technical component of the tests. We also support our pathology clients with interpretation and consultative services using our own specialized team of pathologists for difficult or complex cases and we provide overflow interpretation services when requested by clients.
We are a leading provider of molecular and NGS testing. These tests are interpreted by NeoGenomics’ team of molecular experts and are often ordered in conjunction with other testing modalities. NGS panels are one of our fastest growing testing areas and clients can often receive a significant amount of biomarker information from very limited samples. These comprehensive panels can allow for faster treatment decisions for patients as compared to a series of single-gene molecular tests being ordered sequentially. We have a broad Molecular testing menu and our targeted NeoTYPE panels include genes relevant to a particular cancer type, as well as other complementary tests such as IHC and FISH. In addition, we offer molecular-only NGS targeted and comprehensive panels which combine DNA and RNA into a single work stream in order to report a full spectrum of genomic alterations, including mutations, fusions, copy number variations, and gene expression. This comprehensive menu means that our clients can get most of their oncology testing needs satisfied by our laboratory. This is attractive to our clients as patient samples do not need to be split and then managed across several laboratories. The acquisition of Inivata provided us with oncology liquid biopsy technology capabilities. InVisionFirst®-Lung is a highly sensitive, targeted plasma-based assay for patients with non-small cell lung cancer, and RaDaR® is a liquid biopsy assay designed to detect residual disease and recurrence in plasma samples from patients with solid tumor malignancies. We expect our molecular laboratory and NGS capabilities to be a key growth driver in the coming years.
In addition, we directly serve oncology, dermatology and other clinician practices that prefer to have a direct relationship with a laboratory for cancer-related genetic testing services. We typically serve these types of clients with a comprehensive service offering where we perform both the technical and professional components of the tests ordered. In certain instances, larger clinician practices have begun to internalize pathology interpretation services, and our tech-only service offering allows these larger clinician practices to also participate in the diagnostic process by performing the PC interpretation services on TC testing performed by us. In these instances we will typically provide all of the more complex, molecular testing services.
Advanced Diagnostics
Our Advanced Diagnostics revenue consists of three revenue streams:
•Clinical trials and research;
•Validation laboratory services; and
•Informatics.
Our Advanced Diagnostics segment supports pharmaceutical firms in their drug development programs by supporting various clinical trials and research. This portion of our business often involves working with the pharmaceutical firms (“sponsors”) on study design as well as performing the required testing. Our medical team often advises the sponsor and works closely with them as specimens are received from the enrolled sites. We also work on developing tests that will be used as part of a companion diagnostic to determine patients’ response to a particular drug. As studies unfold, our clinical trials team reports the data and often provides key analysis and insights back to the sponsors.
Our Advanced Diagnostics segment provides comprehensive testing services in support of our pharmaceutical clients’ oncology programs from discovery to commercialization. In biomarker discovery, our aim is to help our customers discover the right content. We help our customers develop a biomarker hypothesis by recommending an optimal platform for molecular screening and backing our discovery tools with the informatics to capture meaningful data. In other pre-clinical and non-clinical work, we can use our platforms to characterize markers of interest. Moving from discovery to development, we seek to help our customers refine their biomarker strategy and, if applicable, develop a companion diagnostic pathway using the optimal technology for large-scale clinical trial testing.
Whether serving as the single contract research organization or partnering with one, our Advanced Diagnostics team provides significant technical expertise, working closely with our customers to support each stage of clinical trial development. Each trial we support comes with rapid turnaround time, dedicated project management and quality assurance oversight. We have experience in supporting submissions to the FDA for companion diagnostics. Our Advanced Diagnostics strategy is focused
44
| NEOGENOMICS, INC. | ||||||||
on helping to bring more effective oncology treatments to market through providing world-class laboratory services in oncology to key pharmaceutical companies in the industry.
We believe that we are well positioned to service Advanced Diagnostics sponsors across the full continuum of the drug development process. Our Advanced Diagnostics team can work with these sponsors during the basic research and development phase as compounds come out of translational research departments, as well as work with clients from Phase I, Phase II and Phase III clinical trials as the sponsors work to demonstrate the efficacy of their drugs. The laboratory biomarker tests that are developed during this process may become CDx tests that will be used on patients to determine if they could respond to a certain therapy. We are able to offer these CDx tests to the market immediately after FDA approval as part of our Day 1 readiness program. This ability helps to speed the commercialization of a drug and can enable Advanced Diagnostics sponsors to reach patients through our broad distribution channel in the Clinical Services segment.
We are committed to connecting patients with life-altering therapies and trials. In carrying out these commitments, we aim to provide transparency and choice to patients regarding the handling and use of their data through our Notice of Privacy Practices, and have invested in leading technologies to ensure the data we maintain is secure at all times. We are continuing to develop and broaden our informatics and data-related tools to leverage our unique market position and oncology expertise to help our stakeholders solve real-world problems such as identifying patients for clinical trials or providing clinical decision support tools for physicians and providers. We also offer testing and informatics tools, such as Trapelo™, to help health care professionals navigate the rapidly evolving field of precision medicine. Trapelo™ is an end-to-end, clinical decision-support platform designed to resolve the complexities of precision oncology – from test ordering to therapy selection to navigating prior authorization.
Critical Accounting Policies and Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates. Our management routinely makes judgments and estimates about the effects of matters that are inherently uncertain. Please refer to Note 2. Summary of Significant Accounting Policies, to our Consolidated Financial Statements for a complete description of our significant accounting policies.
Our critical accounting policies are those where we have made difficult, subjective, or complex judgments in making estimates and/or where these estimates can significantly impact our financial results under different assumptions and conditions. Our critical accounting policies are:
•Goodwill;
•Contingencies; and
•Revenue Recognition and Accounts Receivable.
Goodwill
We evaluate goodwill on an annual basis in the fourth quarter, or more frequently if management believes indicators of impairment exist. Such indicators could include, but are not limited to, (1) a significant adverse change in legal factors or in business climate, (2) unanticipated competition, or (3) an adverse action or assessment by a regulator. We first assess qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, including goodwill. If management concludes that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, management performs a quantitative goodwill impairment test. The quantitative analysis is performed by comparing the fair value of the reporting unit to its carrying value. If the carrying value is greater than the estimate of fair value, an impairment loss will be recognized for the amount in which the carrying amount exceeds the reporting unit’s fair value. We estimate the fair values of our reporting units using a combination of the income, or discounted cash flows approach and the market approach, which utilizes comparable companies’ data.
As of June 30, 2022, we performed a qualitative assessment to determine whether it was more likely than not that the fair values of our reporting units were less than their carrying values. Such qualitative factors included macroeconomic conditions, industry and market considerations, cost factors, overall financial performance and other relevant events. As a result of the qualitative assessment, we determined that due to changes in executive leadership and the sustained decline in our stock price of $12.15 per share as of March 31, 2022 to $8.15 per share as of June 30, 2022, there were indicators that it was more likely than not that the fair values of the reporting units were less than their carrying values. Accordingly, we performed a quantitative analysis and compared our reporting units’ fair values to their carrying values to determine whether goodwill was impaired. We determined the fair values of our reporting units using a combination of the income approach
45
| NEOGENOMICS, INC. | ||||||||
using discounted cash flows and the market approach utilizing comparable companies’ data. The assumptions and estimates, including management’s estimated future revenue growth rates, estimated future margins and discount rates, used in the quantitative analysis were based on management’s best estimate about current and future conditions including projected net revenue from emerging market technologies acquired through the June 2021 acquisition of Inivata. The results of the quantitative analysis showed that the reporting units’ fair values exceeded their carrying values and there was no impairment of the recorded goodwill as of June 30, 2022.
On October 1, 2023, we performed a qualitative assessment to determine whether it was more likely than not that the fair values of our reporting units were less than their carrying values. As a result of the qualitative assessment, we determined that it is not more likely than not that the fair value of our reporting units is less than their carrying amounts.
Contingencies
We accrue contingent losses when estimated impacts of various conditions, situations or circumstances involve uncertain outcomes. Contingent losses are recorded based on management judgment along with internal and external advice from legal counsel and/or technical consultants. Estimated losses from contingencies are recorded when both of the following conditions are met: (i) information available before the financial statements are issued (or available to be issued) indicates that it is probable that an asset has been impaired or a liability has been incurred at the date of the financial statements and (ii) the amount of loss can be reasonably estimated. If some amount within a range of loss appears at the time to be a better estimate than any other amount within the range, that amount shall be accrued. When no amount within the range is a better estimate than any other amount, however, the minimum amount in the range shall be accrued.
Revenue Recognition and Accounts Receivable
Clinical Services
Revenue is recognized when, or as, performance obligations under the terms of a contract are satisfied, which occurs when control of the promised products or services is transferred to a customer. For Clinical Services, our specialized diagnostic services are performed based on an online test order or a written test requisition form. The performance obligation is satisfied and revenues are recognized once the diagnostic services have been performed and the results have been delivered to the ordering physician.
These diagnostic services are billed to various payers, including client direct billing, commercial insurance, Medicare and other government payers, and patients. Revenue is recorded for all payers based on the amount expected to be collected, which considers implicit price concessions. Accounts receivable are reported for all Clinical Services payers based on the amount expected to be collected, which also considers implicit price concessions. Implicit price concessions represent differences between amounts billed and the estimated consideration we expect to receive based on negotiated discounts, historical collection experience, assumptions in payer mix, and other anticipated adjustments, including anticipated payer denials. Collection of consideration we expect to receive typically occurs within 90 to 120 days of billing for commercial insurance, Medicare and other governmental and self-pay patients and within 60 to 90 days of billing for client payers.
The following table reflects our estimate of the breakdown of net clinical revenue by type of payer for the years ended December 31, 2023, 2022 and 2021:
| 2023 | 2022 | 2021 | |||||||||||||||
| Client direct billing | 67 | % | 67 | % | 63 | % | |||||||||||
| Commercial insurance | 18 | % | 17 | % | 19 | % | |||||||||||
| Medicare and other government | 15 | % | 16 | % | 18 | % | |||||||||||
| Total | 100 | % | 100 | % | 100 | % | |||||||||||
Results of Operations for the year ended December 31, 2023 as compared with the year ended December 31, 2022
Revenue
The Company has historically reported its activities in two reportable segments; (1) the Clinical Services segment and (2) the Pharma Services segment. In the second quarter of 2023, the Pharma Services segment was rebranded as the Advanced Diagnostics segment. Clinical Services and Advanced Diagnostics net revenue for the years ended December 31, 2023 and 2022, are as follows (dollars in thousands):
46
| NEOGENOMICS, INC. | ||||||||
| 2023 | 2022 | % Change | |||||||||||||||
| Net revenue: | |||||||||||||||||
| Clinical Services | $ | 495,636 | $ | 418,754 | 18.4 | % | |||||||||||
| Advanced Diagnostics | 96,007 | 90,974 | 5.5 | % | |||||||||||||
| Total net revenue | $ | 591,643 | $ | 509,728 | 16.1 | % | |||||||||||
Consolidated revenue in 2023 increased $81.9 million, or 16.1%, as compared to 2022. Clinical Services revenue increased $76.9 million, or 18.4%, to $495.6 million in 2023 as compared to $418.8 million in 2022. Increases in Clinical Services revenue reflects an increase in test volume, a more favorable test mix, and an increase in average unit price due to strategic reimbursement initiatives.
Advanced Diagnostics revenue increased $5.0 million, or 5.5%, to $96.0 million in 2023 as compared to $91.0 million in 2022, primarily driven by increased volume and higher billings across its portfolio.
Cost of Revenue and Gross Profit
Cost of revenue includes compensation and benefit costs for performing tests, maintenance and/or depreciation of laboratory equipment, rent for laboratory facilities, laboratory reagents, probes and supplies, delivery and courier costs relating to the transportation of specimens to be tested, and amortization for acquired intangible assets.
The consolidated cost of revenue and gross profit metrics for the years ended December 31, 2023 and 2022 are as follows (dollars in thousands):
| 2023 | 2022 | % Change | |||||||||||||||
| Cost of revenue: | |||||||||||||||||
Clinical Services(1)
|
$ | 287,059 | $ | 261,742 | 9.7 | % | |||||||||||
Advanced Diagnostics(2)
|
59,980 | 60,090 | (0.2) | % | |||||||||||||
| Total cost of revenue | $ | 347,039 | $ | 321,832 | 7.8 | % | |||||||||||
| Cost of revenue as a percentage of revenue | 58.7 | % | 63.1 | % | |||||||||||||
| Gross Profit: | |||||||||||||||||
| Clinical Services | $ | 208,577 | $ | 157,012 | 32.8 | % | |||||||||||
| Advanced Diagnostics | 36,027 | 30,884 | 16.7 | % | |||||||||||||
| Total gross profit | $ | 244,604 | $ | 187,896 | 30.2 | % | |||||||||||
| Gross profit margin | 41.3 | % | 36.9 | % | |||||||||||||
_________________
(1) Clinical Services cost of revenue for the twelve months ended December 31, 2023 and December 31, 2022 include $17.3 million and $17.1 million, respectively, of amortization of acquired intangible assets.
(2) Advanced Diagnostics cost of revenue for both the twelve months ended December 31, 2023 and December 31, 2022 include $2.4 million of amortization of acquired intangible assets.
Consolidated cost of revenue increased for the year ended December 31, 2023 when compared to the same period in 2022 primarily due to higher compensation and benefit costs and an increase in supplies expense partially offset by a decrease in professional fees and shipping costs.
Gross profit margin for 2023 was 41.3% compared to 36.9% in 2022. This 4.4% increase is primarily related to increases in revenue partially offset by higher compensation and benefits costs and supplies expense.
General and Administrative Expenses
General and administrative expenses consist of compensation and benefit costs for our executive, billing, finance, human resources, information technology, and other administrative personnel, as well as stock-based compensation. We also allocate professional services, facilities expense, IT infrastructure costs, depreciation, amortization, and other administrative-related costs to general and administrative expenses.
Consolidated general and administrative expenses for the years ended December 31, 2023 and 2022 are as follows (dollars in thousands):
47
| NEOGENOMICS, INC. | ||||||||
| 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||
| General and administrative | $ | 243,101 | $ | 243,356 | $ | (255) | (0.1) | % | |||||||||||||||
| General and administrative as a percentage of revenue | 41.1 | % | 47.7 | % | |||||||||||||||||||
General and administrative expenses decreased $0.3 million in 2023 compared to 2022. This decrease was partially due to a decrease in recruiting expenses of $3.4 million, a decrease in loss on disposals of assets of $1.2 million, a decrease in credit card fees of $0.7 million, and a decrease in non-recurring facilities costs of $0.5 million. These decreases in general and administrative expenses for the year ended December 31, 2023 were partially offset by an increase in travel expenses of $1.7 million, an increase in depreciation and amortization expense of $1.2 million, an increase in compensation and benefit costs of $1.0 million, an increase in equipment expenses of $0.9 million, and an increase in professional fees of $0.7 million.
Research and Development Expenses
Research and development expenses relate to costs of developing new proprietary and non-proprietary genetic tests, including compensation and benefit costs, maintenance of laboratory equipment, laboratory supplies (reagents), and outside consultants and experts assisting our research and development team. Research and development expenses are presented net of research and development tax and expenditure credits from the UK government, which are recognized over the period necessary to match the reimbursement with the related costs when it is probable that the Company has complied with any conditions attached and will receive the reimbursement.
Consolidated research and development expense for the years ended December 31, 2023 and 2022 are as follows (dollars in thousands):
| 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||
| Research and development | $ | 27,309 | $ | 30,326 | $ | (3,017) | (9.9) | % | |||||||||||||||
| Research and development as a percentage of revenue | 4.6 | % | 5.9 | % | |||||||||||||||||||
Research and development expenses decreased $3.0 million in 2023 compared to 2022. This decrease is primarily due to decreases in compensation and benefits costs and professional fees and an increase in research and development tax credits from the UK government.
We anticipate research and development expenditures will increase in the future as we continue to invest in development activities for innovation projects and bringing new tests to market.
Sales and Marketing Expenses
Sales and marketing expenses are primarily attributable to employee-related costs including sales management, sales representatives, sales and marketing consultants, and marketing and customer service personnel.
Consolidated sales and marketing expenses for the years ended December 31, 2023 and 2022, are as follows (dollars in thousands):
| 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||
| Sales and marketing | $ | 70,842 | $ | 67,321 | $ | 3,521 | 5.2 | % | |||||||||||||||
| Sales and marketing as a percentage of revenue | 12.0 | % | 13.2 | % | |||||||||||||||||||
Sales and marketing expenses increased $3.5 million in 2023 compared to 2022. The increase primarily reflects increases in compensation and benefit costs due to increased headcount, an increase in sales commissions, and an increase in travel expenses partially offset by a decrease in professional fees.
We expect higher commissions expense in the coming quarters as our sales representatives generate new business in our business segments. We expect our sales and marketing expenses over the long term to align with changes in revenue and we continue to evaluate the effectiveness of our incentive compensation plans.
Restructuring charges
Consolidated restructuring charges for the years ended December 31, 2023 and 2022 are as follows (dollars in thousands):
| 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||
| Restructuring charges | $ | 11,088 | $ | 4,516 | $ | 6,572 | 145.5 | % | |||||||||||||||
| Restructuring charges as a percentage of revenue | 2.0 | % | 1.0 | % | |||||||||||||||||||
48
| NEOGENOMICS, INC. | ||||||||
Restructuring charges relate to a restructuring program to improve execution and drive efficiency across the organization. Restructuring charges consist of severance and other employee costs, costs for optimizing the Company’s geographic presence, and consulting and other costs.
Restructuring charges increased $6.6 million in 2023 compared to 2022. The increase is primarily due to restructuring activities commencing in the fourth quarter of 2022. Restructuring charges in 2023 consist of $5.6 million in severance and other employee costs, $3.4 million loss on the impairment of facilities and assets, and $2.1 million of consulting and other costs.
In the third quarter of 2023, in response to new incremental information including ongoing negotiations with counterparties, we revised our original restructuring plan cost and timing of approved projects. As a result, we anticipate incurring further restructuring charges extending into 2024. Restructuring activities are ongoing and we expect to incur additional restructuring charges of approximately $6.3 million. We expect these charges will ultimately result in enhanced operational efficiencies as we continue to optimize our geographic presence.
Interest Income
Interest income for the years ended December 31, 2023 and 2022 is as follows (dollars in thousands):
| 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||
| Interest income | $ | (16,902) | $ | (6,075) | $ | (10,827) | 178.2 | % | |||||||||||||||
Interest income increased $10.8 million in 2023 compared to 2022. Interest income includes interest earned on funds held in our cash equivalent and marketable securities accounts. The increase in interest income in 2023 was due to the higher interest rate environment experienced when compared to the same period in 2022.
For further details regarding our investments in marketable securities, please refer to Note 3. Fair Value Measurements in the accompanying notes to the Consolidated Financial Statements.
Interest Expense
Interest expense for the years ended December 31, 2023 and 2022 is as follows (dollars in thousands):
| 2023 | 2022 | $ Change | % Change | ||||||||||||||||||||
| Interest expense | $ | 6,907 | $ | 7,581 | $ | (674) | (8.9) | % | |||||||||||||||
Interest expense decreased $0.7 million in 2023 compared to 2022. Interest expense for the years ended December 31, 2023 and 2022 primarily reflects the effective interest rate on the 2028 Convertible Notes and the 2025 Convertible Notes which is 0.70% and 1.96%, respectively. Interest on the 2028 Convertible Notes and 2025 Convertible Notes began accruing upon issuance and is payable semi-annually.
For further details regarding the convertible notes please refer to Note 7. Debt in the accompanying notes to the Consolidated Financial Statements.
Net Loss
The following table provides the net loss for the years ended December 31, 2023 and 2022, along with the computation of basic and diluted net loss per share (in thousands, except per share amounts):
| 2023 | 2022 | ||||||||||
| Net loss | $ | (87,968) | $ | (144,250) | |||||||
| Basic weighted average shares outstanding | 125,502 | 124,217 | |||||||||
| Diluted weighted average shares outstanding | 125,502 | 124,217 | |||||||||
| Basic net loss per share | $ | (0.70) | $ | (1.16) | |||||||
| Diluted net loss per share | $ | (0.70) | $ | (1.16) | |||||||
49
| NEOGENOMICS, INC. | ||||||||
Non-GAAP Measures
Use of Non-GAAP Financial Measures
In order to provide greater transparency regarding our operating performance, the financial results and financial guidance include the use of certain non-GAAP financial measures that involve adjustments to GAAP results. Non-GAAP financial measures exclude certain income and/or expense items that management believes are not directly attributable to our core operating results and/or certain items that are inconsistent in amounts and frequency, making it difficult to perform a meaningful evaluation of our current or past operating performance. Management believes that the presentation of operating results using non-GAAP financial measures provides useful supplemental information to investors by facilitating the analysis of our core test-level operating results across reporting periods and when comparing those same results to those published by our peers. These non-GAAP financial measures may also assist investors in evaluating future prospects. Management also uses non-GAAP financial measures for financial and operational decision making, planning and forecasting purposes and to manage the business. These non-GAAP financial measures do not replace the presentation of financial information in accordance with U.S. GAAP financial results, should not be considered measures of liquidity, and are unlikely to be comparable to non-GAAP financial measures used by other companies.
Definitions of Non-GAAP Measures
Non-GAAP Adjusted EBITDA
“Adjusted EBITDA” is defined by NeoGenomics as net (loss) income from continuing operations before: (i) interest income and expense, (ii) tax (benefit) or expense, (iii) depreciation and amortization expense, (iv) non-cash stock-based compensation expense, and, if applicable in a reporting period, (v) acquisition and integration related expenses, (vi) CEO transition costs, (vii) restructuring costs, and (viii) other significant or non-operating expenses, net.
The following is a reconciliation of GAAP net loss to Non-GAAP EBITDA and Adjusted EBITDA for the years ended December 31, 2023 and 2022 (dollars in thousands):
| 2023 | 2022 | ||||||||||
| NET LOSS (GAAP) | $ | (87,968) | $ | (144,250) | |||||||
| Adjustments to net loss: | |||||||||||
| Interest income | (16,902) | (6,075) | |||||||||
| Interest expense | 6,907 | 7,581 | |||||||||
| Income tax benefit | (9,129) | (15,092) | |||||||||
| Depreciation | 37,450 | 35,372 | |||||||||
| Amortization of intangibles | 35,133 | 34,058 | |||||||||
| EBITDA (non-GAAP) | (34,509) | (88,406) | |||||||||
| Further Adjustments to EBITDA: | |||||||||||
| Acquisition and integration related expenses | — | 2,479 | |||||||||
| CEO transition costs | 500 | 4,518 | |||||||||
| Non-cash stock-based compensation | 24,633 | 24,672 | |||||||||
| Restructuring charges | 11,088 | 4,516 | |||||||||
Other significant expenses (income), net(3)
|
1,774 | 4,211 | |||||||||
| ADJUSTED EBITDA (non-GAAP) | $ | 3,486 | $ | (48,010) | |||||||
_________________
(3) For the year ended December 31, 2023, other significant (income) expenses, net, includes fees related to a regulatory matter and other non-recurring items. For the year ended December 31, 2022, other significant (income) expenses, net, includes fees related to a regulatory matter, moving costs, a gain on the sale of a building and other non-recurring items.
To date, we have financed our operations primarily through cash generated from operations, public and private sales of debt and equity securities, and bank debt borrowings.
The following table presents a summary of our consolidated cash flows for operating, investing, and financing activities for the years ended December 31, 2023 and 2022, as well as the period ending cash and cash equivalents and working capital (in thousands):
50
| NEOGENOMICS, INC. | ||||||||
| 2023 | 2022 | ||||||||||
| Net cash (used in) provided by: | |||||||||||
| Operating activities | $ | (1,953) | $ | (65,993) | |||||||
| Investing activities | 76,707 | 517 | |||||||||
| Financing activities | 4,554 | 11,829 | |||||||||
| Net change in cash and cash equivalents | 79,308 | (53,647) | |||||||||
| Cash, cash equivalents and restricted cash, beginning of year | 263,180 | 316,827 | |||||||||
| Cash and cash equivalents, end of year | $ | 342,488 | $ | 263,180 | |||||||
Working Capital,(4) end of period
|
$ | 500,508 | $ | 515,359 | |||||||
_________________
(4) Defined as current assets less current liabilities.
Cash Flows from Operating Activities
Cash used in operating activities during the year ended December 31, 2023, was $2.0 million compared to $66.0 million in the same period in 2022. This $64.0 million decrease was primarily driven by our operating results (net loss adjusted for depreciation, amortization of intangibles, and other non-cash charges) which resulted in $57.8 million of lower cash used by operating activities year-over-year, as well as a $6.2 million decrease in cash used resulting from net changes in operating assets and liabilities. The decrease in cash used by operating activities for the year ended December 31, 2023 compared to the same period in 2022 was primarily driven by an improvement in gross profit of $56.7 million.
Cash Flows from Investing Activities
During the year ended December 31, 2023, cash provided by investing activities was $76.7 million, compared to $0.5 million of cash used in investing activities for the same period in 2022. This change was due to a $90.8 million decrease in purchases of marketable securities and a $2.1 million decrease in purchases of property and equipment. These decreases were offset by a $4.7 million decrease in the sales and maturities of marketable securities year-over-year and a $12.1 million decrease in proceeds from assets held for sale which did not occur in 2022.
Cash Flows from Financing Activities
During the year ended December 31, 2023, cash provided by financing activities was $4.6 million compared to $11.8 million for the same period in 2022. The cash provided by financing activities during the year ended December 31, 2023 consisted of $4.6 million for the issuance of common stock net of issuance costs offset by $0.1 million used for the repayment of equipment financing obligations. The primary reason for the decrease in cash provided by financing activities year-over-year was a $8.0 million decrease in cash provided by the issuance of common stock, net due to the timing of stock option exercises for certain former executives.
Liquidity Outlook
As of December 31, 2023, we had $342.5 million in cash and cash equivalents in addition to $72.7 million of marketable securities available to support current operational liquidity needs. We anticipate that the cash on hand, marketable securities, and cash collections are sufficient to fund our near-term capital and operating needs for at least the next 12 months. Operating needs include, but are not limited to, the planned costs to operate our business (including amounts required to fund working capital and capital expenditures, continued research, and development efforts) and potential strategic acquisitions and investments.
Related Party Transactions
Please refer to Note 16. Related Party Transactions, to our Consolidated Financial Statements for a description of our related party transactions.
Capital Expenditures
We forecast capital expenditures in order to execute on our business plan and maintain growth; however, the actual amount and timing of such capital expenditures will ultimately be determined by the volume of business. We currently anticipate that our capital expenditures for the year ended December 31, 2024, will be in the range of $35 million to $40 million. During the
51
| NEOGENOMICS, INC. | ||||||||
year ended December 31, 2023, we purchased, with cash, approximately $28.7 million of capital equipment, software, and leasehold improvements. We have funded and plan to continue funding these capital expenditures with cash and financing.
Recently Adopted Accounting Guidance
Please refer to Note 2. Summary of Significant Accounting Policies, to our Consolidated Financial Statements for a discussion of recently adopted accounting pronouncements and accounting pronouncements pending adoption.
Effects of Inflation
During the years ended December 31, 2023, 2022 and 2021, inflation did not have a material effect on our business. Widely reported inflation has occurred, however, and may be ongoing for the foreseeable future. Depending on the severity and persistence of these inflationary pressures, we could experience, in the future, a negative impact on our financial results. While we anticipate an increasingly uncertain macroeconomic environment in fiscal year 2024, we will continue to mitigate through targeted pricing and various sourcing strategies. We remain optimistic about our growth opportunities in our key markets in fiscal year 2024.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risks, including changes in foreign currency exchange rates.
Interest Rate Risk
In May 2020, we issued $201.3 million aggregate principal amount of the 2025 Convertible Notes. The 2025 Convertible Notes have a fixed annual interest rate of 1.25%; therefore, we do not have economic interest rate exposure with respect to the 2025 Convertible Notes. In January 2021, we issued $345.0 million aggregate principal amount of the 2028 Convertible Notes. The 2028 Convertible Notes have a fixed annual interest rate of 0.25%; therefore, we do not have economic interest rate exposure with respect to the 2028 Convertible Notes. However, the fair value of the 2025 Convertible Notes and 2028 Convertible Notes is exposed to interest rate risk. Generally, the fair market value will increase as interest rates fall and decrease as interest rates rise. In addition, the fair value is affected by our common stock price. The fair value will generally increase as our common stock price increases and will generally decrease as our common stock price declines. We carry the 2025 Convertible Notes and 2028 Convertible Notes at face value less unamortized debt discount and debt issuance costs on our balance sheet, and we present the fair value for required disclosure purposes only.
The primary objective of our investment activities is to preserve principal while at the same time maximizing yields without significantly increasing risk. To achieve this objective, we invest in highly liquid and high-quality U.S. government and other highly credit rated debt securities. Our investments are exposed to market risk due to fluctuations in interest rates, which may affect our interest income and the fair market value of our investments. To minimize our exposure due to adverse shifts in interest rates, we invest in short-term securities with short maturities. If a 1% change in interest rates were to have occurred on December 31, 2023, this change would not have had a material effect on the fair value of our investment portfolio as of that date. Due to the short holding period of our investments, we do not believe that we have a material financial market risk exposure and do not expect our operating results or cash flows to be materially affected by a sudden change in market interest rates. While we believe our marketable securities do not contain excessive risk, we cannot provide absolute assurance that in the future our investments will not be subject to adverse changes in market value.
Foreign Currency Exchange Risk
We have operations in Cambridge, United Kingdom. Our international revenues and expenses denominated in foreign currencies (primarily British Pounds), expose us to the risk of fluctuations in foreign currency exchange rates against the U.S. dollar. We do not hedge foreign currency exchange risks and do not currently believe that these risks are significant.
52
| NEOGENOMICS, INC. | ||||||||
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||||||||
Report of Independent Registered Public Accounting Firm – |
||||||||
53
| NEOGENOMICS, INC. | ||||||||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of NeoGenomics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of NeoGenomics, Inc. and subsidiaries (the "Company") as of December 31, 2023 and 2022, the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows, for each of the three years in the period ended December 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 20, 2024, expressed an unqualified opinion on the Company's internal control over financial reporting.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current-period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Revenue Recognition—Clinical Services—Refer to Notes 2 and 10 to the financial statements
Critical Audit Matter Description
As discussed in Note 10 to the financial statements, revenue for the Company’s clinical services is recognized once the diagnostic services have been performed and the results have been delivered to the ordering physician. Revenue is recorded for all payers based on the amount expected to be collected, which considers implicit price concessions.
Implicit price concessions represent differences between amounts billed and the estimated consideration the Company expects to receive based on negotiated discounts, historical collection experience and other anticipated adjustments, including anticipated payer denials.
54
| NEOGENOMICS, INC. | ||||||||
We identified management’s estimation of implicit price concessions related to clinical services revenue recorded that has not been received in cash as a critical audit matter due to management’s manual process used to determine the estimate, and the significant judgments required by management to estimate payer behavior. This required a high degree of auditor judgment and an increased extent of effort when performing audit procedures to evaluate the reasonableness of management’s assumptions related to expected receipts that were applied in the estimate of implicit price concessions.
How the Critical Audit Matter Was Addressed in the Audit
Our audit procedures related to management’s judgments in the estimate of implicit price concessions included the following, among others:
•We tested the effectiveness of controls over management’s determination of assumptions used to calculate implicit price concessions.
•We tested the methodology used by the Company to estimate implicit price concessions.
•We tested the assumptions used by management to calculate implicit price concessions by:
◦Testing the mathematical accuracy of management’s calculation of implicit price concessions.
◦Testing the historical cash receipts compared to the amounts billed to payers, which are used in the estimate of implicit price concessions, by making selections and agreeing the selected information to source documents.
◦Testing management’s ability to estimate implicit price concessions accurately by comparing recorded net revenue to cash receipts received through January 2024.
◦Evaluating trends in revenue and accounts receivable compared to previous periods to identify any evidence that may contradict management’s assertion regarding implicit price concessions.
/s/ Deloitte & Touche LLP
February 20, 2024
We have served as the Company’s auditor since 2019.
55
| NEOGENOMICS, INC. | ||||||||
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share amounts)
| As of December 31, | |||||||||||
| 2023 | 2022 | ||||||||||
| ASSETS | |||||||||||
| Current assets | |||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Marketable securities, at fair value | |||||||||||
| Accounts receivable, net | |||||||||||
| Inventories | |||||||||||
| Prepaid assets | |||||||||||
| Other current assets | |||||||||||
| Total current assets | |||||||||||
|
Property and equipment (net of accumulated depreciation of
$
|
|||||||||||
| Operating lease right-of-use assets | |||||||||||
| Intangible assets, net | |||||||||||
| Goodwill | |||||||||||
| Other assets | |||||||||||
| Total non-current assets | |||||||||||
| Total assets | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||
| Current liabilities | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued compensation | |||||||||||
| Accrued expenses and other liabilities | |||||||||||
| Current portion of equipment financing obligations | |||||||||||
| Current portion of operating lease liabilities | |||||||||||
| Contract liabilities | |||||||||||
| Total current liabilities | |||||||||||
| Long-term liabilities | |||||||||||
| Convertible senior notes, net | |||||||||||
| Operating lease liabilities | |||||||||||
| Deferred income tax liabilities, net | |||||||||||
| Other long-term liabilities | |||||||||||
| Total long-term liabilities | |||||||||||
| Total liabilities | |||||||||||
Commitments and contingencies (Note 15) |
|||||||||||
Stockholders’ equity |
|||||||||||
|
Common stock, $
and
|
|||||||||||
| Additional paid-in capital | |||||||||||
| Accumulated other comprehensive loss | ( |
( |
|||||||||
| Accumulated deficit | ( |
( |
|||||||||
| Total stockholders’ equity | |||||||||||
| Total liabilities and stockholders’ equity | $ | $ | |||||||||
See the accompanying notes to the Consolidated Financial Statements.
56
| NEOGENOMICS, INC. | ||||||||
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
| For the Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| NET REVENUE | |||||||||||||||||
| Clinical Services | $ | $ | $ | ||||||||||||||
| Advanced Diagnostics | |||||||||||||||||
| Total net revenue | |||||||||||||||||
| COST OF REVENUE | |||||||||||||||||
| GROSS PROFIT | |||||||||||||||||
| Operating expenses: | |||||||||||||||||
| General and administrative | |||||||||||||||||
| Research and development | |||||||||||||||||
| Sales and marketing | |||||||||||||||||
| Restructuring charges | |||||||||||||||||
| Total operating expenses | |||||||||||||||||
| LOSS FROM OPERATIONS | ( |
( |
( |
||||||||||||||
| Interest income | ( |
( |
( |
||||||||||||||
| Interest expense | |||||||||||||||||
| Other expense (income), net | ( |
||||||||||||||||
| Gain on investment in and loan receivable from non-consolidated affiliate, net |
( |
||||||||||||||||
| Loss before taxes | ( |
( |
( |
||||||||||||||
| Income tax benefit | ( |
( |
( |
||||||||||||||
| NET LOSS | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| NET LOSS PER SHARE | |||||||||||||||||
| Basic | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| Diluted | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING |
|||||||||||||||||
| Basic | |||||||||||||||||
| Diluted | |||||||||||||||||
See the accompanying notes to the Consolidated Financial Statements.
57
| NEOGENOMICS, INC. | ||||||||
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
| Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| NET LOSS | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| OTHER COMPREHENSIVE (LOSS) INCOME: | |||||||||||||||||
| Net unrealized gain (loss) on marketable securities, net of tax | ( |
( |
|||||||||||||||
| Total other comprehensive income (loss), net of tax | ( |
( |
|||||||||||||||
| COMPREHENSIVE LOSS | $ | ( |
$ | ( |
$ | ( |
|||||||||||
See the accompanying notes to the Consolidated Financial Statements.
58
| NEOGENOMICS, INC. | ||||||||
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands, except share amounts)
| Common Stock | Additional Paid-In Capital | Accumulated Other Comprehensive (Loss) Income | Accumulated Deficit | |||||||||||||||||||||||||||||||||||
| Shares | Amount | Total | ||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2020 | $ | $ | $ | $ | ( |
$ | ||||||||||||||||||||||||||||||||
| Cumulative-effect adjustment from change in accounting principle | — | — | ( |
— | ( |
|||||||||||||||||||||||||||||||||
| Premiums paid for capped call confirmations | — | — | ( |
— | — | ( |
||||||||||||||||||||||||||||||||
| Issuance of common stock for ESPP | — | — | — | |||||||||||||||||||||||||||||||||||
| Issuance of restricted stock, net of forfeitures | ( |
— | — | ( |
||||||||||||||||||||||||||||||||||
| Issuance of common stock for stock options | — | — | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock - private placement, net of private placement fees | — | — | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock - public offering, net of underwriting discounts | — | — | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock for acquisition | — | — | ||||||||||||||||||||||||||||||||||||
| Stock issuance fees and expenses | — | — | ( |
— | — | ( |
||||||||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Net unrealized loss on marketable securities, net of tax | — | — | — | ( |
— | ( |
||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( |
( |
||||||||||||||||||||||||||||||||
| Balance, December 31, 2021 | $ | $ | $ | ( |
$ | ( |
$ | |||||||||||||||||||||||||||||||
| Issuance of common stock for ESPP | — | — | — | |||||||||||||||||||||||||||||||||||
| Issuance of restricted stock, net of forfeitures | ( |
— | — | ( |
||||||||||||||||||||||||||||||||||
| Issuance of common stock for stock options | — | — | ||||||||||||||||||||||||||||||||||||
| Stock issuance fees and expenses | — | — | ( |
— | — | ( |
||||||||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Net unrealized loss on marketable securities, net of tax | — | — | — | ( |
— | ( |
||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( |
( |
||||||||||||||||||||||||||||||||
| Balance, December 31, 2022 | $ | $ | $ | ( |
$ | ( |
$ | |||||||||||||||||||||||||||||||
| Issuance of common stock for ESPP | — | — | — | |||||||||||||||||||||||||||||||||||
| Issuance of restricted stock, net of forfeitures | ( |
— | ( |
— | — | ( |
||||||||||||||||||||||||||||||||
| Issuance of common stock for stock options | — | — | — | |||||||||||||||||||||||||||||||||||
| Stock issuance fees and expenses | — | — | ( |
— | — | ( |
||||||||||||||||||||||||||||||||
| Stock-based compensation expense | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Net unrealized gain on marketable securities, net of tax | — | — | — | — | ||||||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( |
( |
||||||||||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | ( |
$ | ( |
$ | |||||||||||||||||||||||||||||||
See the accompanying notes to the Consolidated Financial Statements.
59
| NEOGENOMICS, INC. | ||||||||
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| For the Years Ended December 31, | |||||||||||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | |||||||||||||||||
| Net loss | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||||||||
| Depreciation | |||||||||||||||||
| Amortization of intangibles | |||||||||||||||||
| Non-cash stock-based compensation | |||||||||||||||||
| Non-cash operating lease expense | |||||||||||||||||
| Amortization of convertible debt discount | |||||||||||||||||
| Amortization of debt issuance costs | |||||||||||||||||
| Gain on investment in and loan receivable from non-consolidated affiliate, net | ( |
||||||||||||||||
| Interest on loan receivable from non-consolidated affiliate | ( |
||||||||||||||||
| Loss on disposal of assets | |||||||||||||||||
| Gain on sale of assets held for sale | ( |
||||||||||||||||
| Impairment of long-lived assets | |||||||||||||||||
| Write off of COVID-19 PCR testing inventory and equipment | |||||||||||||||||
| Other non-cash items | |||||||||||||||||
| Changes in assets and liabilities, net: | |||||||||||||||||
| Accounts receivable, net | ( |
( |
( |
||||||||||||||
| Inventories | ( |
( |
|||||||||||||||
| Prepaid lease asset | ( |
||||||||||||||||
| Prepaid and other assets | ( |
( |
( |
||||||||||||||
| Operating lease liabilities | ( |
( |
( |
||||||||||||||
| Deferred income tax liabilities, net | ( |
( |
( |
||||||||||||||
| Accrued compensation | |||||||||||||||||
| Accounts payable, accrued and other liabilities | ( |
||||||||||||||||
| Net cash used in operating activities | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| CASH FLOWS FROM INVESTING ACTIVITIES | |||||||||||||||||
| Purchases of marketable securities | ( |
( |
( |
||||||||||||||
| Proceeds from maturities of marketable securities | |||||||||||||||||
| Purchases of property and equipment | ( |
( |
( |
||||||||||||||
| Proceeds from assets held for sale | |||||||||||||||||
| Business acquisitions, net of cash acquired | ( |
||||||||||||||||
| Loan receivable from non-consolidated affiliate | ( |
||||||||||||||||
| Net cash provided by (used in) investing activities | $ | $ | $ | ( |
|||||||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES | |||||||||||||||||
| Repayment of equipment financing obligations | ( |
( |
( |
||||||||||||||
| Issuance of common stock, net | |||||||||||||||||
| Proceeds from issuance of convertible debt, net of issuance costs | |||||||||||||||||
| Premiums paid for capped call transactions | ( |
||||||||||||||||
| Proceeds from equity offering, net of issuance costs | |||||||||||||||||
| Net cash provided by financing activities | $ | $ | $ | ||||||||||||||
| Net change in cash and cash equivalents | $ | $ | ( |
$ | |||||||||||||
| Cash, cash equivalents and restricted cash, beginning of year | $ | $ | $ | ||||||||||||||
| Cash, cash equivalents and restricted cash, end of year | $ | $ | $ | ||||||||||||||
| Supplemental disclosure of cash flow information: | |||||||||||||||||
| Interest paid | $ | $ | $ | ||||||||||||||
| Income taxes paid, net | $ | $ | $ | ||||||||||||||
| Supplemental disclosure of non-cash investing and financing information: | |||||||||||||||||
| Fair value of common stock issued to fund business acquisition | $ | $ | $ | ||||||||||||||
| Purchases of property and equipment included in accounts payable | $ | $ | $ | ||||||||||||||
See the accompanying notes to the Consolidated Financial Statements.
60
| NEOGENOMICS, INC. | ||||||||
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Note 1. Nature of Business
Nature of the Business
NeoGenomics, Inc., a Nevada corporation (the “Parent,” “Company,” or “NeoGenomics”), and its subsidiaries provide a wide range of oncology diagnostic testing and consultative services which includes technical laboratory services and professional interpretation of laboratory test results by licensed physicians who specialize in pathology and oncology. The Company operates a network of cancer-focused testing laboratories in the United States and the United Kingdom.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying Consolidated Financial Statements include the accounts of the Parent and its subsidiaries. All intercompany accounts and balances have been eliminated in consolidation.
Use of Estimates
The Company prepares its Consolidated Financial Statements in conformity with GAAP. These principles require management to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, together with amounts disclosed in the related notes to the Consolidated Financial Statements. Actual results and outcomes may differ from management’s estimates, judgments and assumptions. Significant estimates, judgments and assumptions used in these Consolidated Financial Statements include, but are not limited, to those related to revenues, accounts receivable and related allowances, contingencies, useful lives and recovery of long-term assets and intangible assets, income taxes and valuation allowances, stock-based compensation, business combinations, impairment analysis of goodwill, and restructuring reserves. These estimates, judgments, and assumptions are reviewed periodically and the effects of material revisions in estimates are reflected on the Consolidated Financial Statements prospectively from the date of the change in estimate.
Principles of Consolidation
The Company determines whether investments in affiliates are a Variable Interest Entity (“VIE”) at the start of each new venture and when a reconsideration event has occurred. A reporting entity must consolidate a VIE if that reporting entity has a variable interest (or combination of variable interests) and is determined to be the primary beneficiary. The primary beneficiary has both the power to direct the activities of the VIE that most significantly impact the entity’s economic performance and the obligation to absorb losses or the right to receive benefits from the VIE that could potentially be significant to the VIE.
Segment Reporting
Business Combinations
The Company accounts for acquisitions of entities over which control is obtained that include inputs and processes and have the ability to create outputs as business combinations. The tangible and identifiable intangible assets acquired and liabilities assumed in a business combination are recorded based on their estimated fair values as of the business combination date, including identifiable intangible assets which either arise from a contractual or legal right or are separable from goodwill. The Company bases the estimated fair value of identifiable intangible assets acquired in a business combination on independent third-party valuations that use information and assumptions provided by management, which consider estimates of inputs and assumptions that a market participant would use. Any excess purchase price over the estimated fair value assigned to the net tangible and identifiable intangible assets acquired less liabilities assumed is recorded to goodwill. The use of alternative valuation assumptions, including estimated revenue projections, growth rates, estimated cost savings, cash flows, discount rates, estimated useful lives, and probabilities surrounding the achievement of contingent milestones could result in different
61
| NEOGENOMICS, INC. | ||||||||
purchase price allocations and amortization expense in current and future periods. Transaction costs associated with acquisitions are expensed as incurred in general and administrative expenses in the Consolidated Statements of Operations. Results of operations and cash flows of acquired companies are included in the Company’s operating results from the date of acquisition.
Fair Value of Financial Instruments
The carrying value of cash, certain cash equivalents, accounts receivable, net, other current assets, accounts payable, accrued expenses and other liabilities, and contract liabilities are considered reasonable estimates of their respective fair values due to their short-term nature.
Cash and Cash Equivalents
Marketable Securities
The Company classifies all marketable securities as available-for-sale, including those with maturity dates beyond 12 months, and therefore these securities are classified within current assets on the Consolidated Balance Sheets as they are available to support current operational liquidity needs.
Marketable securities are carried at fair value, with the unrealized holding gains and losses, net of income taxes, reflected in accumulated other comprehensive income until realized. The Company evaluates its marketable securities for other-than-temporary impairment on a quarterly basis. Unrealized losses are charged against net earnings when a decline in fair value is determined to be other-than-temporary. The Company reviews several factors to determine whether a loss is other-than-temporary, such as the length and extent of the fair value decline, the financial condition and near-term prospects of the issuer, and whether there is the intent to sell or will more likely than not be required to sell before the securities’ anticipated recovery. There were no other-than-temporary impairments for the years ended December 31, 2023, 2022 and 2021. Regardless of the intent to sell a security, the Company performs additional analysis on all securities with unrealized losses to evaluate losses associated with the creditworthiness of the security. Credit losses are recorded when the Company does not expect to receive cash flows sufficient to recover the amortized cost basis of a security.
Accounts Receivable, net
Accounts receivable are reported for all Clinical Services payers based on the amount expected to be collected, which considers implicit price concessions. Implicit price concessions represent differences between amounts billed and the estimated consideration the Company expects to receive based on negotiated discounts, historical collection experience, and other anticipated adjustments, including anticipated payer denials.
For Advanced Diagnostics, the Company negotiates billing schedules and payment terms on a contract-by-contract basis which can include payments based on certain milestones being achieved.
Inventories
Inventories consist principally of testing supplies and are valued at lower of cost or net realizable value, using the first-in, first-out method. The Company periodically reviews its inventories for excess or obsolescence and writes-down obsolete or otherwise unmarketable inventories to their estimated net realizable value.
Prepaid Assets
The Company records a prepaid expense for costs paid but not yet incurred. Those expected to be incurred within one year are recorded as prepaid assets within total current assets on the Consolidated Balance Sheets. Any costs expected to be incurred outside of one year are recorded as other assets within total non-current assets on the Consolidated Balance Sheets.
62
| NEOGENOMICS, INC. | ||||||||
Other Current Assets
As of December 31, 2023 and 2022, other current assets consisted primarily of receivables related to research and development (“R&D”) tax credits related to operations in the United Kingdom, contract assets and other non-trade receivables.
Property and Equipment, net
Property and equipment are recorded at cost, net of accumulated depreciation and amortization. Depreciation and amortization are computed on the straight-line basis over the estimated useful lives of the assets. Leasehold improvements are amortized over the shorter of the related lease terms or their estimated useful lives. Costs incurred in connection with the development of internal-use software are capitalized in accordance with the accounting standard for internal-use software, and are amortized over the expected useful life of the software.
The Company periodically reviews the estimated useful lives of property and equipment. Changes to the estimated useful lives are recorded prospectively from the date of the change. Upon retirement or sale, the cost of the assets disposed of, and the related accumulated depreciation, are removed from the accounts and any resulting gain or loss is included in loss from operations. Repairs and maintenance costs are expensed as incurred and are included in cost of revenue, general and administrative expenses or R&D expenses, as appropriate in the Consolidated Statements of Operations.
Leases
The Company leases corporate offices and laboratory spaces throughout the world, all of which are classified as operating leases expiring at various dates and generally having terms ranging from 1 to 20 years. Leases with an initial term of 12 months or less are not recorded on the balance sheet.
Some of the Company’s real estate lease agreements include options to either renew or early terminate the lease. Leases with renewal options allow the Company to extend the lease term typically between 1 and 5 years. When it is reasonably certain that the Company will exercise an option to renew or terminate a lease, these options are considered in determining the classification and measurement of the lease at lease commencement.
Lease liabilities are recorded based on the present value of the future lease payments over the lease term and assessed as of the commencement date. Incentives received from landlords, such as reimbursements for tenant improvements and rent abatement periods, effectively reduce the total lease payments owed for leases.
Certain real estate leases also include executory costs such as common area maintenance (non-lease component), as well as property insurance and property taxes (non-components). Lease payments, which may include lease components, non-lease components and non-components, are included in the measurement of the Company’s lease liabilities to the extent that such payments are either fixed amounts or variable amounts based on a rate or index (fixed in substance), as stipulated in the lease contract. Any actual costs in excess of such amounts are expensed as incurred as variable lease cost.
The Company utilizes its incremental borrowing rate by lease term in order to calculate the present value of its future lease payments when the implicit rates in the leases agreements are not readily determinable. The discount rate represents a risk-adjusted rate on a secured basis and is the rate at which the Company would borrow funds to satisfy the scheduled lease liability payment streams commensurate with the lease term.
Operating lease costs represent fixed lease payments recognized on a straight-line basis over the lease term. Operating lease costs include an immaterial amount of variable lease costs and are recorded in cost of revenue, general and administrative, sales and marketing, and R&D expenses (depending on the nature of the leased asset) in the Consolidated Statements of Operations.
Intangible Assets, net
At December 31, 2023 and 2022 the Company’s intangible assets were comprised of customer relationships, trade names and trademarks, marketing assets, and developed technology.
63
| NEOGENOMICS, INC. | ||||||||
Recoverability and Impairment of Long-Lived Assets
Goodwill
Contingencies
Debt Issuance Costs
Stock-based Compensation
The Company measures compensation expense for stock-based awards to employees, non-employee contracted physicians, and directors based upon the awards’ initial grant-date fair values. Stock-based compensation expense for stock options, restricted stock awards, restricted stock units and performance awards is recorded over the requisite service period in general and administrative expenses on the Consolidated Statements of Operations. For awards with only a service condition, the Company expenses stock-based compensation using the straight-line method over the requisite service period for the entire award. For awards with a market condition, the Company expenses the grant date fair value at the target over the vesting period regardless of the value that the award recipients ultimately receive. The fair values of stock option grants are estimated as of the date of grant by applying the Black-Scholes option valuation model. The fair value of restricted stock with a market condition is estimated at the date of grant using the Monte Carlo simulation model. The Black-Scholes and Monte Carlo models incorporate assumptions as to stock price volatility, the expected life of options or restricted stock, a risk-free interest
64
| NEOGENOMICS, INC. | ||||||||
rate and dividend yield. The fair value of restricted stock without a market condition is estimated using the current market price of the Company’s common stock on the date of grant.
Black-Scholes is affected by the stock price on the date of the grant as well as assumptions regarding a number of highly complex and subjective variables. These variables include the expected term of the option, expected risk-free interest rate, the expected volatility of common stock, and expected dividend yield; each of which is described below. The assumptions for expected term and expected volatility are the two assumptions that significantly affect the grant date fair value.
Expected Term: The expected term of an option is determined using the simplified method under SAB 107 which represents the average between the vesting term and the contractual term. The Company utilizes the simplified method to determine the expected life of the options due to insufficient exercise activity during recent years.
Risk-free Interest Rate: The risk-free interest rate used in the Black-Scholes model is based on the implied yield at the grant date of the U.S. Treasury zero-coupon issue with an equivalent term to the stock-based award being valued. Where the expected term of a stock-based award does not correspond with the term for which a zero coupon interest rate is quoted, the Company uses the nearest interest rate from available maturities.
Expected Stock Price Volatility: The Company uses its own historical weekly volatility because that is more reflective of market conditions.
Dividend Yield: Because the Company has never paid a dividend and does not expect to begin doing so in the foreseeable future, the Company assumed no dividend yield in valuing the stock-based awards.
The fair value of the performance stock units (“PSUs”) granted during the year ended December 31, 2023 was estimated as of the grant date using a Monte Carlo simulation, which requires management to make assumptions regarding risk-free interest rates and volatility of the Company’s stock price. The Monte Carlo simulation incorporates the same assumptions as Black-Scholes as to stock price volatility, the risk-free interest rate and dividend yield. The Company utilized the expected life of the PSUs for the expected term of the award, as the vesting term and contractual term of the awards are identical.
Revenue Recognition
Clinical Services Revenue
The Company’s specialized diagnostic services are performed based on a written test requisition form or an electronic equivalent. The performance obligation is satisfied and revenues are recognized once the diagnostic services have been performed and the results have been delivered to the ordering physician. These diagnostic services are billed to various payers, including client direct billing, commercial insurance, Medicare and other government payers, and patients. Revenue is recorded for all payers based on the amount expected to be collected, which considers implicit price concessions. Implicit price concessions represent differences between amounts billed and the estimated consideration the Company expects to receive based on negotiated discounts, historical collection experience, and other anticipated adjustments, including anticipated payer denials. Collection of consideration the Company expects to receive typically occurs within 90 to 120 days of billing for commercial insurance, Medicare and other governmental and self-pay patients and within 60 to 90 days of billing for client payers.
Advanced Diagnostics Revenue
The Company’s Advanced Diagnostics segment generally enters into contracts with pharmaceutical and biotech customers as well as other CRO to provide research and clinical trial services. Such services also include validation studies and assay development. The Company records revenue on a unit-of-service basis based on the number of units completed towards the satisfaction of a performance obligation. In addition, certain contracts include upfront fees and the revenue for those contracts is recognized over time as services are performed.
Additional offerings within the Advanced Diagnostics portfolio includes Informatics, which involves the licensing of de-identified data to pharmaceutical and biotech customers in the form of either retrospective records or prospective deliveries of data. Informatics revenue is recognized at a point in time upon delivery of retrospective data or over time for prospective data feeds. The Company negotiates billing schedules and payment terms on a contract-by-contract basis, and contract terms generally provide for payments based on a unit-of-service arrangement.
Amounts collected in advance of services being provided are deferred as contract liabilities on the Consolidated Balance Sheets. The associated revenue is recognized and the contract liability is reduced as the contracted services are subsequently performed. Contract assets are established for revenue recognized but not yet billed. These contract assets are reduced once the customer is invoiced and a corresponding receivable is recorded. Additionally, Advanced Diagnostics incurs sales commissions in the process of obtaining contracts with customers. Sales commissions that are payable upon contract award
65
| NEOGENOMICS, INC. | ||||||||
are recognized as assets and amortized over the expected contract term. The amortization of commission expense is based on the weighted average contract duration for all commissionable awards in the respective business in which the commission expense is paid, which approximates the period over which goods and services are transferred to the customer. For offerings with primarily short-term contracts, such as Informatics, the Company applies the practical expedient which allows costs to obtain a contract to be expensed when incurred, if the amortization period of the assets that would otherwise have been recognized is one year or less. Contract assets and capitalized commissions are included in other current assets and other assets on the Consolidated Balance Sheets.
Most contracts are terminable by the customers, either immediately or according to advance notice terms specified within the contracts. All contracts require payment of fees to the Company for services rendered through the date of termination and may require payment for subsequent services necessary to conclude the study or close out the contract.
Cost of Revenue
General and Administrative Expenses
General and administrative expenses consist of payroll and payroll related costs for the Company’s billing, finance, human resources, information technology, and other administrative personnel as well as stock-based compensation. The Company also includes professional services, facilities expense, IT infrastructure costs, depreciation, amortization, and other administrative-related costs in general and administrative expenses in the Consolidated Statements of Operations.
Research and Development Expenses
R&D costs are expensed as incurred. R&D expenses consist of payroll and payroll related costs, laboratory supplies, depreciation of laboratory equipment, and costs for samples to complete validation studies. These expenses are primarily incurred to develop new genetic tests. R&D expenses are presented net of R&D tax and expenditure credits from the UK government, which are recognized over the period necessary to match the reimbursement with the related costs when it is probable that the Company has complied with any conditions attached and will receive the reimbursement.
Sales and Marketing Expenses
Sales and marketing expenses are primarily attributable to employee-related costs including sales management, sales representatives, sales and marketing consultants, and marketing and customer service personnel in the Clinical Services segment. Advertising costs are expensed at the time they are incurred and are deemed immaterial for the years ended December 31, 2023, 2022 and 2021.
Restructuring charges
Income Taxes
We account for income taxes under the asset and liability method, which requires the recognition of deferred tax assets and deferred tax liabilities for the expected future tax consequences of events that have been included in the financial statements. Under this method, we determine deferred tax assets and liabilities on the basis of the differences between financial statement and tax bases of the assets and liabilities by using enacted tax rates in effect for the year in which the differences are expected to reverse. The effect on deferred taxes of a change in tax rates is recognized in income in the period that included the enactment date.
66
| NEOGENOMICS, INC. | ||||||||
We recognize deferred tax assets to the extent that we believe that these assets are more likely than not to be realized. In making such a determination, we consider all positive and negative evidence. If we determine that we would be able to realize our deferred tax assets in the future in excess of their net recorded amount, we would make an adjustment to the valuation allowance, which would reduce the provision for income taxes.
The Company evaluates tax positions that have been taken or are expected to be taken in its tax returns and records a liability for uncertain tax positions, if deemed necessary. The Company follows a two-step approach to recognizing and measuring uncertain tax positions. First, tax positions are recognized if the weight of available evidence indicates that it is more likely than not that the position will be sustained upon examination, including resolution of related appeals or litigation processes, if any. Second, the tax position is measured as the largest amount of tax benefit that has a greater than 50% likelihood of being realized upon settlement.
We recognize interest expense and penalties related to income tax matters, including unrecognized tax benefits, as a component of income tax expense.
Net Loss per Common Share
The Company calculates basic net (loss) income per share attributable to common stockholders by dividing net (loss) income by the weighted-average number of shares of common stock outstanding for the period. Diluted net (loss) income per share is computed using the weighted average number of common shares outstanding during the applicable period, plus the dilutive effect of potential common stock. Potential common stock consists of shares issuable pursuant to stock options and convertible notes, as well as nonvested restricted stock awards and performance stock units which are not considered outstanding with respect to the weighted average common shares outstanding in the calculation of basic net (loss) income per share. Potentially dilutive shares are determined by applying the treasury stock method to the Company’s outstanding stock options and restricted stock awards. Potentially dilutive shares issuable upon conversion of the 0.25 % Convertible Senior Notes due 2028 and the 1.25 % Convertible Senior Notes due 2025 are calculated using the if-converted method.
Recent Accounting Pronouncements
In October 2021, the FASB issued ASU No. 2021-08, Business Combinations (Topic 805), Accounting for Contract Assets and Contract Liabilities from Contracts with Customers (“ASU 2021-08”). This update amends guidance to require that an entity (acquirer) recognize and measure contract assets and contract liabilities acquired in a business combination in accordance with Revenue from Contracts with Customers (Topic 606). At the acquisition date, an acquirer should account for the related revenue contracts in accordance with Topic 606 as if it had originated the contracts. ASU 2021-08 is effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years. Early adoption of the amendments is permitted including adoption in an interim period. If the Company early adopts in an interim period, the Company is required to apply the amendments (1) retrospectively to all business combinations for which the acquisition date occurs on or after the beginning of the fiscal year that includes the interim period of early application and (2) prospectively to all business combinations that occur on or after the date of initial application. The amendments in ASU 2021-08 should be applied prospectively to business combinations occurring on or after the effective date of the amendments. The Company adopted this standard as of January 1, 2023 and there was no impact on its Consolidated Financial Statements.
Accounting Pronouncements Pending Adoption
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures. This update requires entities to consistently categorize and provide greater disaggregation of information in the rate reconciliation and to further disaggregate income taxes paid by jurisdiction. ASU 2023-09 is effective for fiscal years beginning after December 15, 2024, with early adoption permitted. ASU 2023-07 may be applied retrospectively or prospectively. The Company is currently evaluating the planned adoption date and the impact of this standard on its annual disclosures.
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. This update requires entities to disclose significant segment expenses by reportable segment if they are regularly provided to the Chief Operating Decision Maker (CODM) and included in each reported measure of segment profit or loss and requires disclosure of other segment items by reportable segment and a description of its composition. ASU 2023-07 is effective for fiscal years beginning after December 15, 2023, with early adoption permitted. ASU 2023-07 should be applied retrospectively to all prior periods presented in the financial statements. The Company will adopt this pronouncement on January 1, 2024, and is currently evaluating the impact of this standard on its annual disclosures.
67
| NEOGENOMICS, INC. | ||||||||
Note 3. Fair Value Measurements
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. A fair value hierarchy has been established based on three levels of inputs, of which the first two are considered observable and the last unobservable.
Level 1: Quoted prices in active markets for identical assets or liabilities. These are typically obtained from real-time quotes for transactions in active exchange markets involving identical assets.
Level 2: Inputs, other than quoted prices included within Level 1, which are observable for the asset or liability, either directly or indirectly. These are typically obtained from readily-available pricing sources for comparable instruments.
Level 3: Unobservable inputs, where there is little or no market activity for the asset or liability. These inputs reflect the reporting entity’s own assumptions of the data that market participants would use in pricing the asset or liability, based on the best information available in the circumstances.
Assets and Liabilities that are Measured at Fair Value on a Recurring Basis
The Company measures certain financial assets at fair value on a recurring basis, including its marketable securities and certain cash equivalents. The Company considers all securities available-for-sale, including those with maturity dates beyond 12 months, and therefore these securities are classified within current assets on the Consolidated Balance Sheets as they are available to support current operational liquidity needs. The money market accounts are valued based on quoted market prices in active markets. The marketable securities are generally valued based on other observable inputs for those securities (including market corroborated pricing or other models that utilize observable inputs such as interest rates and yield curves) based on information provided by independent third-party pricing entities, except for U.S. Treasury securities which are valued based on quoted market prices in active markets.
The following tables set forth the amortized cost, gross unrealized gains, gross unrealized losses, and fair values of the Company’s marketable securities accounted for as available-for-sale securities as of December 31, 2023 and 2022 (in thousands):
| December 31, 2023 | ||||||||||||||||||||||||||
| Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | |||||||||||||||||||||||
| Financial Assets: | ||||||||||||||||||||||||||
| Short-term marketable securities: | ||||||||||||||||||||||||||
| U.S. Treasury securities | $ | $ | $ | ( |
$ | |||||||||||||||||||||
| Yankee bonds | ( |
|||||||||||||||||||||||||
| Agency bonds | ( |
|||||||||||||||||||||||||
| Municipal bonds | ( |
|||||||||||||||||||||||||
| Commercial paper | ||||||||||||||||||||||||||
| Asset-backed securities | ( |
|||||||||||||||||||||||||
| Corporate bonds | ( |
|||||||||||||||||||||||||
| Total | $ | $ | $ | ( |
$ | |||||||||||||||||||||
68
| NEOGENOMICS, INC. | ||||||||
| December 31, 2022 | ||||||||||||||||||||||||||
| Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | |||||||||||||||||||||||
| Financial Assets: | ||||||||||||||||||||||||||
| Short-term marketable securities: | ||||||||||||||||||||||||||
| U.S. Treasury securities | $ | $ | $ | ( |
$ | |||||||||||||||||||||
| Yankee bonds | ( |
|||||||||||||||||||||||||
| Agency bonds | ( |
|||||||||||||||||||||||||
| Municipal bonds | ( |
|||||||||||||||||||||||||
| Commercial paper | ||||||||||||||||||||||||||
| Asset-backed securities | ( |
|||||||||||||||||||||||||
| Corporate bonds | ( |
|||||||||||||||||||||||||
| Total | $ | $ | $ | ( |
$ | |||||||||||||||||||||
The Company had $1.7 million and $0.9 million of accrued interest receivable at December 31, 2023 and 2022, respectively, included in on its Consolidated Balance Sheets related to its marketable securities. Realized gains or losses were immaterial
The following tables set forth the fair value of available-for-sale marketable securities by contractual maturity at December 31, 2023 and 2022 (in thousands):
| December 31, 2023 | ||||||||||||||||||||||||||
| One Year or Less | Over One Year Through Five Years | Over Five Years | Total | |||||||||||||||||||||||
| Financial Assets: | ||||||||||||||||||||||||||
| Marketable Securities: | ||||||||||||||||||||||||||
| U.S. Treasury securities | $ | $ | $ | $ | ||||||||||||||||||||||
| Yankee bonds | ||||||||||||||||||||||||||
| Agency bonds | ||||||||||||||||||||||||||
| Municipal bonds | ||||||||||||||||||||||||||
| Commercial paper | ||||||||||||||||||||||||||
| Asset-backed securities | ||||||||||||||||||||||||||
| Corporate bonds | ||||||||||||||||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||||||||||||
| December 31, 2022 | ||||||||||||||||||||||||||
| One Year or Less | Over One Year Through Five Years | Over Five Years | Total | |||||||||||||||||||||||
| Financial Assets: | ||||||||||||||||||||||||||
| Marketable Securities: | ||||||||||||||||||||||||||
| U.S. Treasury securities | $ | $ | $ | $ | ||||||||||||||||||||||
| Yankee bonds | ||||||||||||||||||||||||||
| Agency bonds | ||||||||||||||||||||||||||
| Municipal bonds | ||||||||||||||||||||||||||
| Commercial paper | ||||||||||||||||||||||||||
| Asset-backed securities | ||||||||||||||||||||||||||
| Corporate bonds | ||||||||||||||||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||||||||||||
69
| NEOGENOMICS, INC. | ||||||||
The following tables set forth the Company’s cash equivalents and marketable securities accounted for as available-for-sale securities that were measured at fair value on a recurring basis based on the fair value hierarchy as of December 31, 2023 and 2022 (in thousands):
| December 31, 2023 | ||||||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||||||
| Financial Assets: | ||||||||||||||||||||||||||
| Cash equivalents: | ||||||||||||||||||||||||||
| Money market funds | $ | $ | $ | $ | ||||||||||||||||||||||
| Commercial paper | ||||||||||||||||||||||||||
| Marketable securities: | ||||||||||||||||||||||||||
| U.S. Treasury securities | ||||||||||||||||||||||||||
| Yankee bonds | ||||||||||||||||||||||||||
| Agency bonds | ||||||||||||||||||||||||||
| Municipal bonds | ||||||||||||||||||||||||||
| Commercial paper | ||||||||||||||||||||||||||
| Asset-backed securities | ||||||||||||||||||||||||||
| Corporate bonds | ||||||||||||||||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||||||||||||
| December 31, 2022 | ||||||||||||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||||||
| Financial Assets: | ||||||||||||||||||||||||||
| Cash equivalents: | ||||||||||||||||||||||||||
| Money market funds | $ | $ | $ | $ | ||||||||||||||||||||||
| Commercial paper | ||||||||||||||||||||||||||
| Marketable securities: | ||||||||||||||||||||||||||
| U.S. Treasury securities | ||||||||||||||||||||||||||
| Yankee bonds | ||||||||||||||||||||||||||
| Agency bonds | ||||||||||||||||||||||||||
| Municipal bonds | ||||||||||||||||||||||||||
| Commercial paper | ||||||||||||||||||||||||||
| Asset-backed securities | ||||||||||||||||||||||||||
| Corporate bonds | ||||||||||||||||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||||||||||||
There were no transfers of financial assets or liabilities into or out of Level 1, Level 2, or Level 3 for the years ended December 31, 2023 and 2022.
Assets and Liabilities that are Measured at Fair Value on a Nonrecurring Basis
The carrying value of cash, certain cash equivalents, accounts receivable, net, other current assets, accounts payable, accrued expenses and other liabilities, and contract liabilities are considered reasonable estimates of their respective fair values at December 31, 2023 and 2022 due to their short-term nature.
The Company also measures certain non-financial assets at fair value on a nonrecurring basis, primarily intangible assets, goodwill, long-lived assets in connection with periodic evaluations for potential impairment. The Company estimates the fair value of these assets using primarily unobservable inputs and as such, these are considered Level 3 fair value measurements.
70
| NEOGENOMICS, INC. | ||||||||
Note 4. Property and Equipment, Net
Property and equipment consisted of the following at December 31, 2023 and 2022 (in thousands):
| 2023 | 2022 |
Estimated Useful
Lives in Years
|
|||||||||||||||
| Equipment | $ | $ | |||||||||||||||
| Leasehold improvements | |||||||||||||||||
| Furniture and fixtures | |||||||||||||||||
| Computer hardware and office equipment | |||||||||||||||||
| Computer software | |||||||||||||||||
| Construction in progress | — | ||||||||||||||||
| Subtotal | |||||||||||||||||
| Less: accumulated depreciation | ( |
( |
|||||||||||||||
| Property and equipment, net | $ | $ | |||||||||||||||
In 2021, the Company committed to selling the Carlsbad facility and the associated land and concluded that these assets met the held for sale criteria. The Company sold this property and associated land for proceeds of $12.1 million, net of closing costs, in 2022. For the year ended December 31, 2022, a net gain on the sale of this property and associated land of $2.0 million is included in general and administrative expenses on the Consolidated Statements of Operations.
Depreciation expense for the years ended December 31, 2023, 2022 and 2021, was as follows (in thousands):
| 2023 | 2022 | 2021 | |||||||||||||||
| Cost of revenue | $ | $ | $ | ||||||||||||||
| General and administrative | |||||||||||||||||
| Research and development | |||||||||||||||||
| Total depreciation | $ | $ | $ | ||||||||||||||
Note 5. Leases
As of December 31, 2023, the maturities of the operating lease liabilities and a reconciliation to the present value of lease liabilities were as follows (in thousands):
| Remaining Lease Payments | |||||
| 2024 | $ | ||||
| 2025 | |||||
| 2026 | |||||
| 2027 | |||||
| 2028 | |||||
| Thereafter | |||||
| Total remaining lease payments | |||||
| Less: imputed interest | ( |
||||
| Total operating lease liabilities | |||||
| Less: current portion | ( |
||||
| Long-term operating lease liabilities | $ | ||||
| Weighted-average remaining lease term (in years) | |||||
| Weighted-average discount rate | % | ||||
71
| NEOGENOMICS, INC. | ||||||||
The following summarizes additional supplemental data related to the operating leases for the years ended December 31, 2023 and 2022 (in thousands):
| 2023 | 2022 | |||||||||||||
| Operating lease costs | $ | $ | ||||||||||||
| Right-of-use assets obtained in exchange for operating lease liabilities | $ | $ | ||||||||||||
| Cash paid for operating leases | $ | $ | ||||||||||||
The Company entered into $12.5 million of contractually binding minimum lease payments for a lease that commenced in November 2023. This amount is included in the above tables and relates to the lease of a laboratory facility in Durham, North Carolina.
Note 6. Goodwill and Intangible Assets
The following table summarizes the changes in the carrying amount of goodwill by segment as of December 31, 2023 and 2022 (in thousands):
| 2023 | 2022 | ||||||||||
| Clinical Services | $ | $ | |||||||||
| Advanced Diagnostics | |||||||||||
| Total | $ | $ | |||||||||
Intangible assets consisted of the following as of December 31, 2023 and 2022 (in thousands):
| 2023 | |||||||||||||||||||||||
| Amortization Period (years) |
Cost |
Accumulated
Amortization
|
Net | ||||||||||||||||||||
| Customer Relationships | $ | $ | $ | ||||||||||||||||||||
| Developed Technology | |||||||||||||||||||||||
| Marketing Assets | |||||||||||||||||||||||
| Trademarks | |||||||||||||||||||||||
| Trade Name | |||||||||||||||||||||||
| Trademark - Indefinite lived | — | — | |||||||||||||||||||||
| Total | $ | $ | $ | ||||||||||||||||||||
| 2022 | |||||||||||||||||||||||
| Amortization Period (years) |
Cost |
Accumulated
Amortization
|
Net | ||||||||||||||||||||
| Customer Relationships | $ | $ | $ | ||||||||||||||||||||
| Developed Technology | |||||||||||||||||||||||
| Marketing Assets | |||||||||||||||||||||||
| Trademarks | |||||||||||||||||||||||
| Trade Name | |||||||||||||||||||||||
| Trademark - Indefinite lived | — | — | |||||||||||||||||||||
| Total | $ | $ | $ | ||||||||||||||||||||
For the years ended December 31, 2023, 2022 and 2021, amortization on the Consolidated Statements of Operations was recorded as follows (in thousands):
72
| NEOGENOMICS, INC. | ||||||||
| 2023 | 2022 | 2021 | |||||||||||||||
| Amortization recorded in: | |||||||||||||||||
| Cost of revenue | $ | $ | $ | ||||||||||||||
| General and administrative | |||||||||||||||||
| Total amortization | $ | $ | $ | ||||||||||||||
As of December 31, 2023, the estimated amortization expense related to amortizable intangible assets for each of the five following years and thereafter is as follows (in thousands):
| 2024 | $ | |||||||
| 2025 | ||||||||
| 2026 | ||||||||
| 2027 | ||||||||
| 2028 | ||||||||
| Thereafter | ||||||||
| Total | $ | |||||||
Note 7. Debt
The following table summarizes long-term debt, net, at December 31, 2023 and 2022 (in thousands):
| 2023 | 2022 | ||||||||||
| Principal | $ | $ | |||||||||
| Unamortized debt discount | ( |
( |
|||||||||
| Unamortized debt issuance costs | ( |
( |
|||||||||
Total |
|||||||||||
| Principal | |||||||||||
| Unamortized debt discount | ( |
( |
|||||||||
| Unamortized debt issuance costs | ( |
( |
|||||||||
Total |
|||||||||||
| Equipment financing obligations | |||||||||||
| Total debt | |||||||||||
| Less: Current portion of equipment financing obligations | ( |
||||||||||
| Total long-term debt, net | $ | $ | |||||||||
At December 31, 2023, the estimated fair value (Level 2) of the 0.25 % Convertible Senior Notes due 2028 and the 1.25 % Convertible Senior Notes due 2025 was $262.4 million and $197.3 million, respectively. At December 31, 2022, the estimated fair value (Level 2) of the 0.25 % Convertible Senior Notes due 2028 and the 1.25 % Convertible Senior Notes due 2025 was $218.2 million and $169.6 million, respectively.
2028 Convertible Senior Notes
On January 11, 2021, the Company completed the sale of $345.0 million of Convertible Senior Notes with a stated interest rate of 0.25 % and a maturity date of January 15, 2028 (the “2028 Convertible Notes”), unless earlier converted, redeemed, or repurchased. The 2028 Convertible Notes were issued at a discounted price of 97.0 % of their principal amount. The total net proceeds from the issuance of the 2028 Convertible Notes and exercise of the over-allotment option was approximately $334.4 million, which includes approximately $10.6 million of discounts, commissions and offering expenses paid by the Company. On January 11, 2021, the Company entered into an indenture (the “2021 Indenture”), with U.S. Bank National Association, as trustee (the “Trustee”), governing the 2028 Convertible Notes. The Company used a portion of the net
73
| NEOGENOMICS, INC. | ||||||||
proceeds from the Offerings to enter into capped call transactions (as described below under the heading “Capped Call Transactions”).
Prior to September 15, 2027, noteholders may convert their 2028 Convertible Notes at their option, only in the following circumstances: (1) during any calendar quarter commencing after the calendar quarter ending on June 30, 2021 (and only during such calendar quarter), if the last reported sale price of the common stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding calendar quarter is greater than or equal to 130.0 % of the conversion price on each applicable trading day; (2) during the five business day period after any five consecutive trading day period in which the trading price per $1,000 principal amount of 2028 Convertible Notes for each trading day of the measurement period was less than 98.0 % of the product of the last reported sale price of the Company’s common stock and the conversion rate on each such trading day; (3) if the Company calls any or all of the notes for redemption, at any time prior to the close of business on the scheduled trading day immediately preceding the redemption date; or (4) upon the occurrence of specified corporate events. On or after September 15, 2027 until the close of business on the second business day immediately preceding the maturity date, noteholders may convert their 2028 Convertible Notes at any time, regardless of the foregoing circumstances.
The last reported sales price of the Company’s common stock was not greater than or equal to 130.0 % of the conversion price of the 2028 Convertible Notes on at least 20 of the last 30 consecutive trading days of the quarter ended September 30, 2023. Based on the terms of the 2028 Convertible Notes, the holders could not have converted all or a portion of their 2028 Convertible Notes in the fourth quarter of 2023. The last reported sales price of the Company’s common stock was not greater than or equal to 130.0 % of the conversion price of the 2028 Convertible Notes on at least 20 of the last 30 consecutive trading days of the quarter ended December 31, 2023. Based on the terms of the 2028 Convertible Notes, the holders cannot convert all or a portion of their 2028 Convertible Notes in the first quarter of 2024. When a conversion notice is received, the Company has the option to pay or deliver cash, shares of the Company’s common stock, or a combination thereof. As the Company is not required to settle the 2028 Convertible Notes in cash, the 2028 Convertible Notes are classified as long-term debt as of December 31, 2023. As of December 31, 2023, the Company had not received any conversion notices.
Upon conversion, the Company will pay or deliver, as applicable, cash, shares of common stock or a combination of cash and shares of common stock, at its election. The initial conversion rate for the 2028 Convertible Notes is 15.1172 shares of common stock per $1,000 in principal amount of 2028 Convertible Notes, equivalent to an initial conversion price of approximately $66.15 per share of common stock. The conversion rate is subject to adjustment as described in the 2021 Indenture. In addition, following certain corporate events that occur prior to the maturity date as described in the 2021 Indenture, the Company will pay a make-whole premium by increasing the conversion rate for a holder who elects to convert its 2028 Convertible Notes in connection with such a corporate event in certain circumstances. The value of the 2028 Convertible Notes, if converted, does not exceed the principal amount based on a closing stock price of $16.18 on December 31, 2023.
The Company may not redeem the 2028 Convertible Notes prior to January 20, 2025. The Company may redeem for cash all or any portion of the 2028 Convertible Notes, at its option, on or after January 20, 2025 if the last reported sale price of its common stock has been at least 130.0 % of the conversion price then in effect for at least 20 trading days (whether or not consecutive) during any 30 consecutive trading day period (including the last trading day of such period) ending on, and including, the trading day immediately preceding the date of notice by the Company of redemption at a redemption price equal to 100.0 % of the principal amount of the 2028 Convertible Notes to be redeemed, plus accrued and unpaid interest to, but excluding, the redemption date. No sinking fund is provided for the 2028 Convertible Notes.
If an event involving bankruptcy, insolvency or reorganization events with respect to the Company occurs, then the principal amount of, and all accrued and unpaid interest on, all of the 2028 Convertible Notes then outstanding will immediately become due and payable. If any other default event occurs and is continuing, then noteholders of at least 25.0 % of the aggregate principal amount of the 2028 Convertible Notes then outstanding, by notice to the Company, may declare the principal amount of, and all accrued and unpaid interest on, all of the 2028 Convertible Notes then outstanding to become due and payable immediately. If the Company undergoes a Fundamental Change (as defined in the 2021 Indenture), then noteholders may require the Company to repurchase their 2028 Convertible Notes at a cash repurchase price equal to the principal amount of the 2028 Convertible Notes to be repurchased, plus accrued and unpaid interest, if any, to, but excluding, the Fundamental Change Repurchase Date (as defined in the 2021 Indenture).
The 2028 Convertible Notes are the Company’s senior, unsecured obligations and will be equal in right of payment with its existing and future senior, unsecured indebtedness, senior in right of payment to its existing and future indebtedness that is expressly subordinated to the 2028 Convertible Notes and effectively junior to its existing and future secured indebtedness, to the extent of the value of the collateral securing that indebtedness. The 2028 Convertible Notes will be structurally subordinated to all existing and future indebtedness and other liabilities, including trade payables, of its subsidiaries.
74
| NEOGENOMICS, INC. | ||||||||
The interest expense recognized on the 2028 Convertible Notes includes $0.9 million, $1.5 million and $34,000 for the contractual coupon interest, the amortization of the debt discount and the amortization of the debt issuance costs, respectively, for the year ended December 31, 2023. The interest expense recognized on the 2028 Convertible Notes includes $0.9 million, $1.5 million and $34,000 for the contractual coupon interest, the amortization of the debt discount and the amortization of the debt issuance costs, respectively, for the year ended December 31, 2022. The effective interest rate on the 2028 Convertible Notes is 0.70 %, which includes the interest on the 2028 Convertible Notes and amortization of the debt discount and debt issuance costs. The 2028 Convertible Notes bear interest at a rate of 0.25 % per annum, payable semi-annually in arrears on January 15 and July 15 of each year, which began on July 15, 2021.
Capped Call Transactions
In connection with the 2028 Convertible Notes offering, on January 11, 2021, the Company entered into separate, privately negotiated convertible note hedge transactions (collectively, the “Capped Call Transactions”) with option counterparties pursuant to capped call confirmations at a cost of approximately $29.3 million. As the Capped Call Transactions meet certain accounting criteria, the Capped Call Transactions were classified as equity, are not accounted for as derivatives and were recorded as a reduction of the Company’s additional paid-in capital in the accompanying Consolidated Financial Statements. The Capped Call Transactions are not part of the terms of the 2028 Convertible Notes and will not affect any holders’ rights under the 2028 Convertible Notes. The Capped Call Transactions cover, subject to customary anti-dilution adjustments, the number of shares of the Company’s common stock that initially underlie the 2028 Convertible Notes. The number of shares underlying the Capped Call Transactions is 5.2 million.
The cap price of the Capped Call Transactions is initially $85.75 per share of the Company’s common stock, which represents a premium of 75.0 % over the public offering price of the common stock in the 2021 Common Stock Offering, which was $49.00 per share, and is subject to certain adjustments under the terms of the Capped Call Transactions.
By entering into the Capped Call Transactions, the Company expects to reduce the potential dilution to its common stock (or, in the event a conversion of the 2028 Convertible Notes is settled in cash, to reduce its cash payment obligation) in the event that, at the time of conversion of the 2028 Convertible Notes, its common stock price exceeds the conversion price of the 2028 Convertible Notes.
2025 Convertible Senior Notes
On May 4, 2020, the Company completed the sale of $201.3 million of convertible senior notes with a stated interest rate of 1.25 % and a maturity date of May 1, 2025 (the “2025 Convertible Notes”), unless earlier converted, redeemed, or repurchased. The 2025 Convertible Notes were issued at a discounted price of 97.0 % of their principal amount. The total net proceeds from the issuance of the 2025 Convertible Notes and exercise of the over-allotment option were approximately $194.5 million, which includes approximately $6.9 million of discounts, commissions and offering expenses paid by the Company. On May 4, 2020, the Company entered into an indenture (the “2020 Indenture”), with U.S. Bank National Association, as trustee (the “Trustee”), governing the 2025 Convertible Notes.
Prior to February 1, 2025, noteholders may convert their 2025 Convertible Notes at their option, only in the following circumstances: (1) during any calendar quarter commencing after the calendar quarter ending on September 30, 2020 (and only during such calendar quarter), if the last reported sale price of the common stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on, and including, the last trading day of the immediately preceding calendar quarter is greater than or equal to 130.0 % of the conversion price on each applicable trading day; (2) during the five business day period after any five consecutive trading day period in which the trading price per $1,000 principal amount of 2025 Convertible Notes for each trading day of the measurement period was less than 98.0 % of the product of the last reported sale price of the Company’s common stock and the conversion rate on each such trading day; (3) if the Company calls any or all of the notes for redemption, at any time prior to the close of business on the scheduled trading day immediately preceding the redemption date; or (4) upon the occurrence of specified corporate events. On or after February 1, 2025 until the close of business on the business day immediately preceding the maturity date, noteholders may convert their 2025 Convertible Notes at any time, regardless of the foregoing circumstances.
The last reported sales price of the Company’s common stock was not greater than or equal to 130.0 % of the conversion price of the 2025 Convertible Notes on at least 20 of the last 30 consecutive trading days of the quarter ended September 30, 2023. Based on the terms of the 2025 Convertible Notes, the holders could not have converted all or a portion of their 2025 Convertible Notes in the fourth quarter of 2023. The last reported sales price of the Company’s common stock was not greater than or equal to 130.0 % of the conversion price of the 2025 Convertible Notes on at least 20 of the last 30 consecutive trading days of the quarter ended December 31, 2023. Based on the terms of the 2025 Convertible Notes, the holders cannot convert all or a portion of their 2025 Convertible Notes in the first quarter of 2024. When a conversion notice is received, the
75
| NEOGENOMICS, INC. | ||||||||
Company has the option to pay or deliver cash, shares of the Company’s common stock, or a combination thereof. As the Company is not required to settle the 2025 Convertible Notes in cash, the 2025 Convertible Notes are classified as long-term debt as of December 31, 2023 and 2022. As of December 31, 2023, the Company had not received any conversion notices.
Upon conversion, the Company will pay or deliver, as applicable, cash, shares of common stock or a combination of cash and shares of common stock, at its election. The initial conversion rate for the 2025 Convertible Notes is 27.5198 shares of common stock per $1,000 in principal amounts of 2025 Convertible Notes, equivalent to an initial conversion price of approximately $36.34 per share of common stock. The conversion rate is subject to adjustment as described in the 2020 Indenture. In addition, following certain corporate events that occur prior to the maturity date as described in the 2020 Indenture, the Company will pay a make-whole premium by increasing the conversion rate for a holder who elects to convert its 2025 Convertible Notes in connection with such a corporate event in certain circumstances. The value of the 2025 Convertible Notes, if converted, does not exceed the principal amount based on a closing stock price of $16.18 on December 31, 2023.
The Company may not redeem the 2025 Convertible Notes prior to May 6, 2023. The Company may redeem for cash all or any portion of the 2025 Convertible Notes, at its option, on or after May 6, 2023 if the last reported sale price of its common stock has been at least 130.0 % of the conversion price then in effect for at least 20 trading days (whether or not consecutive) during any 30 consecutive trading day period (including the last trading day of such period) ending on, and including, the trading day immediately preceding the date of notice by the Company of redemption at a redemption price equal to 100.0 % of the principal amount of the 2025 Convertible Notes to be redeemed, plus accrued and unpaid interest to, but excluding, the redemption date. No sinking fund is provided for the 2025 Convertible Notes.
If an event involving bankruptcy, insolvency or reorganization events with respect to the Company occurs, then the principal amount of, and all accrued and unpaid interest on, all of the 2025 Convertible Notes then outstanding will immediately become due and payable. If any other default event occurs and is continuing, then noteholders of at least 25.0 % of the aggregate principal amount of the 2025 Convertible Notes then outstanding, by notice to the Company, may declare the principal amount of, and all accrued and unpaid interest on, all of the 2025 Convertible Notes then outstanding to become due and payable immediately. If the Company undergoes a “fundamental change” as defined in the 2020 Indenture, then noteholders may require the Company to repurchase their 2025 Convertible Notes at a cash repurchase price equal to the principal amount of the 2025 Convertible Notes to be repurchased, plus accrued and unpaid interest, if any, to, but excluding, the fundamental change repurchase date.
The 2025 Convertible Notes are the Company’s senior, unsecured obligations and will be equal in right of payment with its existing and future senior, unsecured indebtedness, senior in right of payment to its existing and future indebtedness that is expressly subordinated to the 2025 Convertible Notes and effectively junior to its existing and future secured indebtedness, to the extent of the value of the collateral securing that indebtedness. The 2025 Convertible Notes will be structurally subordinated to all existing and future indebtedness and other liabilities, including trade payables, of its subsidiaries.
The interest expense recognized on the 2025 Convertible Notes includes $2.5 million, $1.2 million and $0.2 million, for the contractual coupon interest, the amortization of the debt discount and the amortization of the debt issuance costs, respectively for the year ended December 31, 2023. The interest expense recognized on the 2025 Convertible Notes includes $2.5 million, $1.2 million and $0.1 million, for the contractual coupon interest, the amortization of the debt discount and the amortization of the debt issuance costs, respectively for the year ended December 31, 2022. The effective interest rate on the 2025 Convertible Notes is 1.96 %, which includes the interest on the 2025 Convertible Notes and amortization of the debt discount and debt issuance costs. The 2025 Convertible Notes bear interest at a rate of 1.25 % per annum, payable semi-annually in arrears on May 1 and November 1 of each year, which began on November 1, 2020.
76
| NEOGENOMICS, INC. | ||||||||
Maturities of Long-Term Debt
Maturities of long-term debt at December 31, 2023 are summarized as follows (in thousands):
| Total Long-Term Debt | ||||||||||||||||||||
| 2024 | $ | $ | $ | |||||||||||||||||
| 2025 | ||||||||||||||||||||
| 2026 | ||||||||||||||||||||
| 2027 | ||||||||||||||||||||
| 2028 | ||||||||||||||||||||
| Thereafter | ||||||||||||||||||||
| Total Debt | ||||||||||||||||||||
| Less: Current portion of long-term debt | ||||||||||||||||||||
| Less: Unamortized debt discount | ( |
( |
( |
|||||||||||||||||
| Less: Unamortized debt issuance costs | ( |
( |
( |
|||||||||||||||||
| Long-term debt, net | $ | $ | $ | |||||||||||||||||
Note 8. Equity Transactions
Underwritten Public Equity Offerings
On January 6, 2021, the Company entered into an underwriting agreement relating to the issuance and sale of 4,081,632 shares of the Company’s common stock, $0.001 par value per share (the “2021 Common Stock Offering”). The price to the public in this offering was $49.00 per share. The net proceeds to the Company from the 2021 Common Stock Offering were approximately $189.9 million, after deducting underwriting discounts, commissions and other offering expenses of approximately $10.1 million.
Under the terms of the underwriting agreement, the Company also granted the Underwriters a 30 -day option to purchase up to 612,244 additional shares of common stock at the public offering price, less underwriting discounts and commissions. On January 6, 2021, the underwriters exercised their option in full and purchased all 612,244 shares. The net proceeds related to the option exercise were approximately $28.4 million, after deducting underwriting discounts, commissions and other offering expenses of approximately $1.6 million.
Common Stock Issued for Acquisition
On April 7, 2021, the Company completed the acquisition of a 100 % ownership interest in Intervention Insights, Inc. d/b/a Trapelo Heath (“Trapelo”). In connection with this acquisition the Company issued 597,712 shares of common stock as consideration for the acquisition of Trapelo in April 2021.
Private Placement Transaction
On June 18, 2021, the Company completed a private placement (“Private Placement”) to certain accredited investors of an aggregate of 4,444,445 shares of the Company’s common stock at a price of $45.00 per share. The net proceeds to the Company from the Private Placement were approximately $189.9 million, after deducting fees to the placement agents and other offering expenses of approximately $10.1 million.
Note 9. Stock-Based Compensation
Equity Incentive Plan
Effective May 25, 2023, the Company adopted the NeoGenomics, Inc. 2023 Equity Incentive Plan (the “2023 Plan”) as approved by the Board of Directors on March 28, 2023 and the Company’s stockholders on May 25, 2023. The 2023 Plan replaced the NeoGenomics, Inc. Amended and Restated Equity Incentive Plan, as most recently amended and subsequently approved by the stockholders on May 25, 2017 (the “Prior Plan”). The 2023 Plan allows for the award of equity incentives including stock options, stock appreciation rights, restricted stock awards, restricted stock units, performance shares, performance units, and other stock-based awards to certain employees, directors, or officers of, or key non-employee advisers or consultants, including contracted physicians to the Company or its subsidiaries. The 2023 Plan provides that the maximum aggregate number of shares of the Company’s common stock reserved and available for issuance under the 2023 Plan is
77
| NEOGENOMICS, INC. | ||||||||
The Company recorded approximately $24.6 million, $24.7 million and $22.5 million for stock-based compensation in general and administrative expenses on the Consolidated Statements of Operations for the years ended December 31, 2023, 2022 and 2021 respectively.
Stock Options
As of December 31, 2023 and 2022, a total of approximately 7.6 million and 4.9 million shares, respectively, were available for future option and stock awards under the 2023 Plan and the Prior Plan, respectively. Options typically expire after 7 or 10 years and generally vest over 3 or 4 years. Each grant’s expiration, vesting, and exercise price provisions are determined at the time the awards are granted by the Compensation Committee of the Board of Directors.
The fair value of each stock award granted during the years ended December 31, 2023, 2022 and 2021 were estimated as of the grant date using a Black-Scholes model. Weighted average assumptions used during the years ended December 31, 2023, 2022 and 2021 are as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Expected term (in years) | |||||||||||||||||
| Risk-free interest rate (%) | |||||||||||||||||
| Expected volatility (%) | |||||||||||||||||
| Dividend yield (%) | |||||||||||||||||
| Weighted average fair value/share at grant date | $ |
$ |
$ |
||||||||||||||
The status of the stock options are summarized as follows:
|
Number
of Shares
|
Weighted
Average Exercise
Price
|
Weighted-Average Remaining Contract Term (in years) |
Aggregate Intrinsic Value (in thousands) |
||||||||||||||||||||
Outstanding at December 31, 2020 |
$ | $ | |||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | ( |
||||||||||||||||||||||
| Forfeited | ( |
||||||||||||||||||||||
Outstanding at December 31, 2021 |
|||||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | ( |
||||||||||||||||||||||
| Forfeited | ( |
||||||||||||||||||||||
Outstanding at December 31, 2022 |
|||||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | ( |
||||||||||||||||||||||
| Forfeited | ( |
||||||||||||||||||||||
Outstanding at December 31, 2023 |
|||||||||||||||||||||||
Exercisable at December 31, 2023 |
|||||||||||||||||||||||
Vested and expected to vest at December 31, 2023 |
|||||||||||||||||||||||
The total cash proceeds received from the exercise of stock options were approximately $3.0 million, $10.3 million and $13.7 million for the years ended December 31, 2023, 2022 and 2021, respectively. The total fair value of option shares vested during the years ended December 31, 2023, 2022 and 2021 was approximately $6.2 million, $8.3 million and $11.7 million, respectively.
The Company recognizes stock-based compensation expense using the straight-line basis over the awards’ requisite service periods. Stock compensation expense related to stock options for the years ended December 31, 2023, 2022 and 2021 was approximately $9.9 million, $8.1 million and $11.6 million, respectively, and is included in general and administrative expenses in the Consolidated Statements of Operations. As of December 31, 2023, there was approximately $12.8 million of
78
| NEOGENOMICS, INC. | ||||||||
total unrecognized stock-based compensation cost related to nonvested stock options granted under the 2023 Plan, the Form of Stand-Alone Inducement Restricted Stock Agreement and Form of Stand-Alone Inducement Stock Option Agreement entered into by the Company and Mr. Christopher M. Smith (collectively, the “CEO Inducement Award”), and the Form of Stand-Alone Inducement Restricted Stock Agreement and Form of Stand-Alone Inducement Stock Option Agreement entered into by the Company and Mr. Jeffrey S. Sherman (collectively, the “CFO Inducement Award”). This cost is expected to be recognized over a weighted-average period of 1.7 years.
Restricted Stock Awards
The number of shares and weighted average grant date fair values of restricted nonvested common stock at the beginning and end of 2023, 2022 and 2021, as well as stock awards granted, vested, and forfeited during the year were as follows:
|
Number of
Restricted
Shares
|
Weighted Average
Grant Date
Fair Value
|
||||||||||
Nonvested at December 31, 2020 |
$ | ||||||||||
| Granted | |||||||||||
| Vested | ( |
||||||||||
| Forfeited | ( |
||||||||||
Nonvested at December 31, 2021 |
|||||||||||
| Granted | |||||||||||
| Vested | ( |
||||||||||
| Forfeited | ( |
||||||||||
Nonvested at December 31, 2022 |
|||||||||||
| Granted | |||||||||||
| Vested | ( |
||||||||||
| Forfeited | ( |
||||||||||
Nonvested at December 31, 2023 |
|||||||||||
The total fair value of restricted stock vested during the years ended December 31, 2023, 2022 and 2021 was approximately $8.7 million, $13.7 million and $7.0 million, respectively.
Stock compensation expense related to restricted stock for the years ended December 31, 2023, 2022 and 2021 was approximately $12.3 million, $15.5 million, and $9.8 million, respectively, and is included in general and administrative expenses in the Consolidated Statements of Operations. As of December 31, 2023, there was approximately $15.3 million of total unrecognized stock-based compensation cost related to nonvested restricted stock granted under the 2023 Plan, the CEO Inducement Award and the CFO Inducement Award. This cost is expected to be recognized over a weighted-average period of 1.8 years.
Performance-Based Restricted Stock Units
The Company granted 305,105 PSUs subject to a market condition to certain of its executives with an aggregate grant date fair value of approximately $6.7 million for the year ended December 31, 2023. The number of shares awarded will be subject to adjustment based on the achievement of a TSR performance target. If the TSR performance target is achieved, the awards will vest at the end of the three-year requisite service period so long as the employee remains employed with the Company through the applicable vesting date. Compensation cost for the PSUs is recognized straight-line over the requisite service period, regardless of when, if ever, the market condition is satisfied.
The Company recognized approximately $1.4 million of stock-based compensation related to the PSUs in general and administrative expenses on the Consolidated Statements of Operations for the year ended December 31, 2023, respectively. There were no
A summary of the PSU activity under the Company’s plans for the year ended December 31, 2023 is as follows:
79
| NEOGENOMICS, INC. | ||||||||
| Number of Stock Units | Weighted Average Grant Date Fair Value | |||||||||||||
Nonvested at December 31, 2022 |
$ | |||||||||||||
| Granted | ||||||||||||||
| Vested | ||||||||||||||
| Forfeited | ||||||||||||||
Nonvested at December 31, 2023 |
||||||||||||||
The fair value of each PSU granted during the year ended December 31, 2023 was estimated as of the grant date using a Monte Carlo simulation with the following assumptions:
| 2023 | |||||
| Expected term (in years) | |||||
| Risk-free interest rate (%) | |||||
| Expected volatility (%) | |||||
| Dividend yield (%) | |||||
| Weighted average grant date fair value per share | $ |
||||
As of December 31, 2023, there was approximately $5.2 million of unrecognized stock-based compensation expense related to nonvested PSUs that will be recognized over a weighted-average period of approximately 2.4 years.
Modifications of Stock Option and Restricted Stock Awards
For the year ended December 31, 2023, the Culture and Compensation Committee of the Company’s Board of Directors approved the accelerated vesting of 101,937 previously granted time-vesting stock option awards and 61,746 previously granted time-vesting restricted stock awards upon the exit of certain officers of the Company. The Company accounted for the effects of the stock awards as modifications, and recognized $0.9 million of incremental stock-based compensation upon acceleration, which consisted of $0.3 million and $0.6 million for the acceleration of stock option awards and restricted stock awards, respectively, for the year ended December 31, 2023. These amounts are included in stock compensation expense for the year ended December 31, 2023 and are recorded as general and administrative expenses in the Company’s Consolidated Statements of Operations.
Employee Stock Purchase Plan
The Company sponsors an Employee Stock Purchase Plan (“ESPP”), under which eligible employees can purchase common stock at a 15.0 % discount from the fair market value. Stock-based compensation expense related to the ESPP for the years ended December 31, 2023, 2022 and 2021 was approximately $1.0 million, $1.0 million and $1.1 million, respectively. Shares issued pursuant to this plan were 326,697 , 415,450 and 112,094 for each of the years ended December 31, 2023, 2022 and 2021, respectively.
Note 10. Revenue Recognition
Clinical Services Revenue
The Company’s specialized diagnostic services are performed based on a written test requisition form or electronic equivalent. The performance obligation is satisfied and revenues are recognized at the point in time the diagnostic services have been performed and the results have been delivered to the ordering physician. These diagnostic services are billed to various payers, including client direct billing, commercial insurance, Medicare and other government payers, and patients. Revenue is recorded for all payers based on the amount expected to be collected, which considers implicit price concessions. Implicit price concessions represent differences between amounts billed and the estimated consideration the Company expects to receive based on negotiated discounts, historical collection experience, and other anticipated adjustments, including anticipated payer denials.
80
| NEOGENOMICS, INC. | ||||||||
Advanced Diagnostics Revenue
The Company’s Advanced Diagnostics segment generally enters into contracts with pharmaceutical and biotech customers as well as other CROs to provide research and clinical trial services. Such services also include validation studies and assay development. The Company records revenue on a unit-of-service basis based on the number of units completed towards the satisfaction of a performance obligation. In addition, certain contracts include upfront fees and the revenue for those contracts is recognized over time as services are performed.
Additional offerings within the Advanced Diagnostics portfolio includes Informatics, which involves the licensing of de-identified data to pharmaceutical and biotech customers in the form of either retrospective records or prospective deliveries of data. Informatics revenue is recognized at a point in time upon delivery of retrospective data or over time for prospective data feeds. The Company negotiates billing schedules and payment terms on a contract-by-contract basis, and contract terms generally provide for payments based on a unit-of-service arrangement.
Amounts collected in advance of services being provided are deferred as contract liabilities on the Consolidated Balance Sheets. The associated revenue is recognized and the contract liability is reduced as the contracted services are subsequently performed. Contract assets are established for revenue recognized but not yet billed. These contract assets are reduced once the customer is invoiced and a corresponding receivable is recorded. Additionally, Advanced Diagnostics incurs sales commissions in the process of obtaining contracts with customers. Sales commissions that are payable upon contract award are recognized as assets and amortized over the expected contract term. The amortization of commission expense is based on the weighted average contract duration for all commissionable awards in the respective business in which the commission expense is paid, which approximates the period over which goods and services are transferred to the customer. For offerings with primarily short-term contracts, such as Informatics, the Company applies the practical expedient which allows costs to obtain a contract to be expensed when incurred, if the amortization period of the assets that would otherwise have been recognized is one year or less. Contract assets and capitalized commissions are included in other current assets and other assets on the Consolidated Balance Sheets.
Most contracts are terminable by the customers, either immediately or according to advance notice terms specified within the contracts. All contracts require payment of fees to the Company for services rendered through the date of termination and may require payment for subsequent services necessary to conclude the study or close out the contract.
The following table summarizes the values of contract assets, capitalized commissions, and contract liabilities for Advanced Diagnostics as of December 31, 2023 and 2022 (in thousands):
| 2023 | 2022 | ||||||||||
Current contract assets(1)
|
$ | $ | |||||||||
Long-term contract assets(2)
|
|||||||||||
| Total contract assets | $ | $ | |||||||||
Current capitalized commissions(1)
|
$ | $ | |||||||||
Long-term capitalized commissions(2)
|
|||||||||||
| Total capitalized commissions | $ | $ | |||||||||
| Current contract liabilities | $ | $ | |||||||||
Long-term contract liabilities(3)
|
|||||||||||
| Total contract liabilities | $ | $ | |||||||||
(1) Recorded within other current assets on the Consolidated Balance Sheets.
(2) Recorded within other assets on the Consolidated Balance Sheets.
Revenue recognized for the years ended December 31, 2023, 2022 and 2021, related to contract liability balances outstanding at the beginning of each year was $6.4 million, $5.2 million, and $4.4 million, respectively. Amortization of capitalized commissions for the years ended December 31, 2023, 2022 and 2021 were $1.0 million, $0.9 million and $1.1 million respectively.
81
| NEOGENOMICS, INC. | ||||||||
Disaggregation of Revenue
The Company considered various factors for both its Clinical Services and Advanced Diagnostics segments in determining appropriate levels of homogeneous data for its disaggregation of revenue; including the nature, amount, timing, and uncertainty of revenue and cash flows. Clinical Services categories align with the types of customers due to similarities of billing method, level of reimbursement, and timing of cash receipts. Unbilled amounts are accrued and allocated to payer categories based on historical experience. In future periods actual billings by payer category may differ from accrued amounts. Advanced Diagnostics relate to contracts with large pharmaceutical and biotech customers as well as other CROs. Because the nature, timing, and uncertainty of revenue and cash flows are similar and primarily driven by individual contract terms Advanced Diagnostics revenue is not further disaggregated.
The following table details the disaggregation of net revenue for both the Clinical Services and Advanced Diagnostics Segments for the years ended December 31, 2023, 2022 and 2021 (in thousands):
| 2023 | 2022 | 2021 | ||||||||||||||||||
| Clinical Services: | ||||||||||||||||||||
| Client direct billing | $ | $ | $ | |||||||||||||||||
| Commercial insurance | ||||||||||||||||||||
| Medicare and other government | ||||||||||||||||||||
| Self-pay | ||||||||||||||||||||
| Total Clinical Services | ||||||||||||||||||||
| Advanced Diagnostics | ||||||||||||||||||||
| Total net revenue | $ | $ | $ | |||||||||||||||||
Note 11. Restructuring
In 2022, the Company embarked on a restructuring program to improve execution and drive efficiency across the organization. This program is a framework for identifying, prioritizing and executing operational improvements. Restructuring charges incurred consist of severance and other employee costs, costs for optimizing the Company’s geographic presence (“Facility Footprint Optimization”), and consulting and other costs.
The following table summarizes the costs associated with the Company’s restructuring activities for the year ended December 31, 2023 (in thousands):
| Severance and Other Employee Costs | Facility Footprint Optimization | Consulting and Other Costs | Total | ||||||||||||||||||||
| Balance as of December 31, 2022 | $ | $ | $ | $ | |||||||||||||||||||
| Restructuring charges incurred | |||||||||||||||||||||||
| Impairment of facility-related assets | |||||||||||||||||||||||
Cash payments and other adjustments(1)
|
( |
( |
( |
( |
|||||||||||||||||||
| Balance as of December 31, 2023 | $ | $ | $ | $ | |||||||||||||||||||
| Current liabilities | $ | ||||||||||||||||||||||
| Long-term liabilities | |||||||||||||||||||||||
| $ | |||||||||||||||||||||||
(1) Other adjustments include non-cash asset charges related to Facility Footprint Optimization costs.
In the third quarter of 2023, in response to new incremental information including ongoing negotiations with counterparties, the Company revised its original restructuring plan, cost and timing of approved projects. As a result, the Company anticipates incurring further restructuring charges extending into 2024. The Company expects these charges will ultimately result in enhanced operational efficiencies as it continues to optimize its geographic presence.
82
| NEOGENOMICS, INC. | ||||||||
Note 12. Income Taxes
(Loss) income before income tax expense (benefit) for the years ended December 31, 2023, 2022 and 2021 is as follows (in thousands):
| 2023 | 2022 | 2021 | |||||||||||||||
| (Loss) income before income tax expense (benefit): | |||||||||||||||||
| Domestic | $ | ( |
$ | ( |
$ | ||||||||||||
| Foreign | ( |
( |
( |
||||||||||||||
| Total | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| Income tax expense (benefit) | |||||||||||||||||
| Current: | |||||||||||||||||
| Federal | $ | $ | ( |
$ | |||||||||||||
| State | |||||||||||||||||
| Foreign | |||||||||||||||||
| Total current tax expense | $ | $ | $ | ||||||||||||||
| Deferred: | |||||||||||||||||
| Federal | $ | ( |
$ | $ | ( |
||||||||||||
| State | ( |
||||||||||||||||
| Foreign | ( |
( |
( |
||||||||||||||
| Total deferred tax benefit | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| Total tax benefit | $ | ( |
$ | ( |
$ | ( |
|||||||||||
A reconciliation of the differences between the effective tax rate and the federal statutory tax rate for the years ended December 31, 2023, 2022 and 2021 is as follows:
| 2023 | 2022 | 2021 | |||||||||||||||
| Federal statutory tax rate | % | % | % | ||||||||||||||
| State income taxes, net of federal income tax benefit | % | % | % | ||||||||||||||
| Transaction Costs | ( |
% | ( |
% | ( |
% | |||||||||||
| Penalties | % | ( |
% | ( |
% | ||||||||||||
| Compensation expense | ( |
% | ( |
% | ( |
% | |||||||||||
| Inivata acquisition fair value adjustment | % | % | % | ||||||||||||||
| Capped call interest | % | % | % | ||||||||||||||
| Tax credits | % | % | % | ||||||||||||||
| Return to provision and other deferred tax adjustments | ( |
% | ( |
% | % | ||||||||||||
| Foreign tax rate differential | % | % | % | ||||||||||||||
| Enacted Rate Changes | % | % | % | ||||||||||||||
| Other, net | ( |
% | ( |
% | ( |
% | |||||||||||
| Valuation allowance | ( |
% | ( |
% | ( |
% | |||||||||||
| Effective tax rate | % | % | % | ||||||||||||||
83
| NEOGENOMICS, INC. | ||||||||
At December 31, 2023 and 2022, deferred income tax assets and liabilities consisted of the following (in thousands):
| 2023 | 2022 | ||||||||||
| Deferred tax assets: | |||||||||||
| Accrued compensation | |||||||||||
| Net operating loss carry-forwards | |||||||||||
| Tax credits | |||||||||||
| Stock-based compensation | |||||||||||
| Operating lease liabilities | |||||||||||
| Interest expense | |||||||||||
| Convertible debt discount | |||||||||||
| Research expenditures | |||||||||||
| Other | |||||||||||
| Gross deferred tax assets | |||||||||||
| Less: valuation allowance | ( |
( |
|||||||||
| Total deferred tax assets | $ | $ | |||||||||
| Deferred tax liabilities: | |||||||||||
| Operating lease right-of-use assets | $ | ( |
$ | ( |
|||||||
| Intangible assets | ( |
( |
|||||||||
| Property and equipment | ( |
( |
|||||||||
| Total deferred tax liabilities | $ | ( |
$ | ( |
|||||||
| Net deferred income tax liabilities | $ | ( |
$ | ( |
|||||||
At December 31, 2023, the Company has federal net operating loss carry forwards of approximately $276.0 million, foreign net operating loss carryforwards of approximately $217.3 million, including $180.1 million in the United Kingdom, and state net operating loss carry forwards of approximately $180.8 million. Federal net operating loss (“NOLs”) carry forwards will begin to expire in 2030. Under the Tax Act, as modified by the CARES Act, the Company’s federal NOLs generated in tax years ending after December 31, 2017 may be carried forward indefinitely, however, the deductibility of such federal net NOLs in tax years beginning after December 31, 2020, is limited to 80% of taxable income. State tax NOLs began to expire in 2023. NOLs in Switzerland and China begin to expire in 2024 and 2025, respectively, if not utilized in future periods. The NOLs in Singapore and the United Kingdom do not expire. As of December 31, 2023, the Company has federal R&D credit carryforwards of approximately $8.6 million that begin to expire in 2036 and state research and investment credit carryforwards of approximately $5.4 million that do not expire.
An ownership change of more than 50 percent could result in a limitation of the use of net operating loss carryforwards and credit carryforwards under IRC Section 382, IRC Section 383, and the regulations thereunder. Based on a completed formal study, there were no ownership changes in prior periods that would materially limit the use of the Company’s net operating loss carryforwards and credit carryforwards under IRC Section 382 and IRC Section 383.
Management assesses the recoverability of its deferred tax assets as of the end of each quarter, weighing all positive and negative evidence, and is required to establish and maintain a valuation allowance for these assets if it is more likely than not that some or all of the deferred tax assets will not be realized. The weight given to the evidence is commensurate with the extent to which the evidence can be objectively verified. If negative evidence exists, positive evidence is necessary to support a conclusion that a valuation allowance is not needed. As of December 31, 2023 and 2022, management determined that sufficient positive evidence did not exist and concluded that it is more likely than not that a valuation allowance is required against deferred tax assets. Accordingly, management established a valuation allowance of $67.8 million related to the Company’s domestic operations, a valuation of $1.5 million related to the Company's United Kingdom operations, and a full valuation allowance of $6.9 million related to the Company’s China, Switzerland and Singapore operations.
The Company files income tax returns in the United States, as well as Singapore, Switzerland, China, United Kingdom and in various state jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment. For U.S. federal purposes, the Company has open tax years ended December 31, 2011 to December 31, 2023. For the United Kingdom the Company has open tax years ended December 31, 2021 and December 31, 2023.
84
| NEOGENOMICS, INC. | ||||||||
The Company applied the accounting standard for uncertain tax positions and recognizes the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the more likely than not threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority. Increases or decreases to the unrecognized tax benefits could result from management’s belief that a position can or cannot be sustained upon examination based on subsequent information or potential lapse of the applicable statute of limitation for certain tax positions.
The following are the unrecognized tax benefits as of December 31, 2023, 2022, and 2021 (in thousands):
| 2023 | 2022 | 2021 | ||||||||||||
| Unrecognized tax benefits - January 1 | $ | $ | $ | |||||||||||
| Increases in prior year positions | ||||||||||||||
| Increases in tax positions taken in current year | ||||||||||||||
| Statute expirations | ( |
|||||||||||||
| Unrecognized tax benefits - December 31 | $ | $ | $ | |||||||||||
Due to the valuation allowance, the majority of unrecognized tax benefits at December 31, 2023, if recognized, would not impact the Company’s effective tax rate. The interest and penalties related to the unrecognized tax benefit are immaterial. Interest and tax penalties related to unrecognized tax benefits are included in income tax expense. Although the timing and outcome of audit settlements are uncertain, it is unlikely there will be a significant reduction of the uncertain tax benefits in the next twelve months.
Note 13. Net Loss per Share
The Company presents both basic earnings per share (“EPS”) and diluted EPS. Basic EPS excludes potential dilution and is computed by dividing net loss by the weighted-average number of common shares outstanding for the period. Diluted EPS reflects the potential dilution that could occur if stock options were exercised, stock awards vested and if the 2028 Convertible Notes and 2025 Convertible Notes were converted. The potential dilution from stock awards is accounted for using the treasury stock method based on the average market value of the Company’s common stock. The potential dilution from conversion of the 2028 Convertible Notes and 2025 Convertible Notes is accounted for using the if-converted method, which requires that all of the shares of the Company’s common stock issuable upon conversion of the 2028 Convertible Notes and the 2025 Convertible Notes will be included in the calculation of diluted EPS assuming conversion of the 2028 Convertible Notes and the 2025 Convertible Notes at the beginning of the reporting period (or at time of issuance, if later).
The following table shows the calculations for the years ended December 31, 2023, 2022 and 2021 (in thousands, except per share amounts):
| 2023 | 2022 | 2021 | |||||||||||||||
| NET LOSS | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| Basic weighted average common shares outstanding | |||||||||||||||||
| Diluted weighted average shares outstanding | |||||||||||||||||
| Basic net loss per share | $ | ( |
$ | ( |
$ | ( |
|||||||||||
| Diluted net loss per share | $ | ( |
$ | ( |
$ | ( |
|||||||||||
The following potential dilutive shares were excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive for the years ended December 31, 2023, 2022 and 2021:
| 2023 | 2022 | 2021 | |||||||||||||||
| Stock options | |||||||||||||||||
| Restricted stock awards | |||||||||||||||||
| 2025 Convertible Notes | |||||||||||||||||
| 2028 Convertible Notes | |||||||||||||||||
85
| NEOGENOMICS, INC. | ||||||||
In addition, 246,856 shares of PSU awards are excluded from the computation of diluted EPS for the year ended December 31, 2023, respectively, as the contingency had not been satisfied.
The Capped Call Transactions are not reflected in diluted net loss per share as they are anti-dilutive. For further details on the Capped Call Transactions, please refer to Note 7. Debt.
Note 14. Defined Contribution Plan
The Company maintains a defined-contribution 401(k) retirement plan covering substantially all U.S. based employees (as defined). The Company’s employees may make voluntary contributions to the plan, subject to limitations based on IRS regulations and compensation. The Company matches 100.0 % of every dollar contributed up to 3.0 % of the respective employee’s compensation and an additional 50.0 % of every dollar contributed on the next 2 % of compensation (4.0 % maximum Company match). Matching contributions were approximately $7.3 million, $7.1 million and $6.1 million during the years ended December 31, 2023, 2022 and 2021, respectively, and are recorded in cost of revenue and operating expenses in the Consolidated Statements of Operations.
Note 15. Commitments and Contingencies
Purchase Commitments
The Company has agreements in place to purchase a specified level of reagents from certain vendors. Typically, the Company can cancel contracts with suppliers without penalties. For those contracts that are not cancelable without penalties, there are termination fees and costs or commitments for continued spending that the Company is obligated to pay to a supplier under each contract’s termination period before such contract can be cancelled. These purchase commitments expire in 2025. The purchase commitments as of December 31, 2023, are as follows (in thousands):
| Years ending December 31, | |||||
| 2024 | $ | ||||
| 2025 | |||||
| Total purchase commitments | $ | ||||
Legal Proceedings
On January 20, 2021, Natera, Inc. filed a patent infringement complaint against the Company’s newly-acquired subsidiary Inivata Limited and its subsidiary Inivata, Inc. in U.S. District Court for the district of Delaware, alleging Inivata’s InVisionFirst®-Lung cancer diagnostic test of infringing two patents. Natera then filed a second patent infringement complaint on December 20, 2022 against Inivata Limited and Inivata, Inc. alleging that RaDaR® minimal residual disease test infringes one patent. The case is in discovery and the jury trial has been scheduled for October 6, 2025.
On July 28, 2023, Natera filed a complaint in the Middle District of North Carolina alleging NeoGenomics' RaDaR test infringes on two patents. On July 31, 2023, Natera moved for a preliminary injunction. On December 27, 2023, the district court issued a preliminary injunction against RaDaR®. Natera posted a $10 million bond with the court on January 12, 2024. The court's initial determination was that Natera, Inc. demonstrated a likelihood that products using RaDaR® technology infringe one Natera, Inc. patent. The order specifically allows patients already using RaDaR® to continue their use. In addition, the order explicitly allows research projects and studies that are in progress, as well as clinical trials that are in progress or have been approved, to continue. On December 28, 2023, NeoGenomics appealed the preliminary injunction to the Federal Circuit. The appeal was docketed at the Federal Circuit on January 4, 2024. On February 5, 2024, NeoGenomics filed an Emergency Motion to Stay the Preliminary Injunction pending Appeal and a Motion to Expedite the appeal. The Federal Circuit granted expedited briefing of the appeal with oral arguments scheduled for March 29, 2024. The Company intends to vigorously pursue its appeal of the preliminary injunction. The infringement case is in discovery and the jury trial has been scheduled for March 10, 2025.
The Company believes that it has good and substantial defenses to the claims alleged in these suits, but there is no guarantee that the Company will prevail. At the time of filing the outcome of these matters is not estimable or probable.
On December 16, 2022, a purported shareholder class action captioned Daniel Goldenberg v. NeoGenomics, Inc., Douglas VanOort, Mark Mallon, Kathryn McKenzie, and William Bonello was filed in the United States District Court for the Southern District of New York, naming the Company and certain of the Company’s current and former officers as defendants. This lawsuit was filed by a stockholder who claims to be suing on behalf of anyone who purchased or otherwise acquired the Company’s securities between February 27, 2020 and April 26, 2022. The lawsuit alleges that material
86
| NEOGENOMICS, INC. | ||||||||
misrepresentations and/or omissions of material fact were made in the Company’s public disclosures in violation of Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. The alleged improper disclosures relate to statements regarding the Company’s menu of tests, business operations and compliance with health care laws and regulations. The plaintiff seeks unspecified monetary damages on behalf of the putative class and an award of costs and expenses, including attorney’s fees and expert fees. On April 27, 2023, a shareholder of the Company filed a shareholder derivative action on behalf of the Company captioned Puskarich v. VanOort, et al. in Clark County Nevada, naming certain of the Company’s current and former officers and directors as defendants. The allegations are substantially similar to the allegations asserted in the Goldenberg action. Substantially similar shareholder derivative actions were subsequently filed in Lee County, Florida and in the United States District Court for the Southern District of New York, captioned Wong v. VanOort, et al. and Mellema v. VanOort, et al., respectively. The Company believes that it has valid defenses to the claims alleged in the lawsuits, but there is no guarantee that the Company will prevail. At the time of filing the outcome of these matters are not estimable or probable.
Regulatory Matter
With the assistance of outside counsel, the Company voluntarily conducted an internal investigation that focused on the compliance of certain consulting and service agreements with federal healthcare laws and regulations, including those relating to fraud, waste and abuse. Based on this internal investigation, the Company voluntarily notified the OIG of the Company’s internal investigation in November 2021. The Company’s interactions with regulatory authorities and the Company’s related review of this matter are ongoing. The Company has a reserve of $11.2
Note 16. Related Party Transactions
The Company has Advanced Diagnostics contracts with HOOKIPA Pharma, Inc., an entity with whom a director of the Company, Michael A. Kelly, was a director until April 2023. In connection with these contracts, the Company recognized $0.4 million, $0.4 million and $0.5 million of revenue in the Consolidated Statements of Operations for the years ended December 31, 2023, 2022, and 2021, respectively.
87
| NEOGENOMICS, INC. | ||||||||
Note 17. Segment Information
The Company has historically reported its activities in two reportable segments, (1) Clinical Services and (2) Pharma Services. In the second quarter of 2023, the Pharma Services segment was rebranded as the Advanced Diagnostics segment.
The financial information reviewed by the CODM includes revenues, cost of revenue, and gross profit for both reportable segments. Assets, operating expenses, loss from operations, and net loss are not presented at the segment level as that information is not used by the CODM. For further details regarding segment reporting, please refer to Note 2. Summary of Significant Accounting Policies.
The following table summarizes segment information for the years ended December 31, 2023, 2022 and 2021 (in thousands):
| 2023 | 2022 | 2021 | |||||||||||||||
| Net revenue: | |||||||||||||||||
| Clinical Services | $ | $ | $ | ||||||||||||||
| Advanced Diagnostics | |||||||||||||||||
| Total net revenue | |||||||||||||||||
| Cost of revenue: | |||||||||||||||||
Clinical Services(1)
|
|||||||||||||||||
Advanced Diagnostics(2)
|
|||||||||||||||||
| Total cost of revenue | |||||||||||||||||
| Gross profit: | |||||||||||||||||
| Clinical Services | |||||||||||||||||
| Advanced Diagnostics | |||||||||||||||||
| Total gross profit | |||||||||||||||||
(1) Clinical Services cost of revenue for the years ended December 31, 2023 and December 31, 2022 include $17.3 million and $17.1 million, respectively, of amortization of acquired intangible assets.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2023. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2023, our disclosure controls and procedures were (1) effective, in that they were designed to ensure that material information relating to us, and information required to be disclosed in our reports to the SEC, including our consolidated subsidiaries, is made known to our Chief Executive Officer and Chief Financial Officer by others within those entities, particularly during the period in which this report was being prepared, as appropriate, to allow timely discussions and decisions regarding required disclosure therein; and (2) effective, in that they provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms.
Management’s Report on Internal Control over Financial Reporting
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) or 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, our principal executive and principal financial officer and effected by the Company’s Board of Directors,
88
| NEOGENOMICS, INC. | ||||||||
management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures: (1) that pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, however, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control—Integrated Framework (“2013 Framework”). Based on this assessment, management, with the participation of our Chief Executive Officer and Chief Financial Officer, concluded that, as of December 31, 2023, our internal control over financial reporting was effective based on those criteria at the reasonable assurance level.
Deloitte & Touche LLP audited the effectiveness of the Company’s internal control over financial reporting as of December 31, 2023, as stated in their report, which is included in this annual report on Form 10-K.
Changes in Internal Control over Financial Reporting
During the fourth quarter of 2023, we continued to monitor and evaluate the design and operating effectiveness of key controls. There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that materially affected or are reasonably likely to materially affect internal control over financial reporting.
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the stockholders and the Board of Directors of NeoGenomics, Inc.
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of NeoGenomics, Inc. and subsidiaries (the “Company”) as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework (2013) issued by COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated financial statements as of and for the year ended December 31, 2023, of the Company and our report dated February 20, 2024, expressed an unqualified opinion on those financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
89
| NEOGENOMICS, INC. | ||||||||
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Deloitte & Touche LLP
San Diego, California
February 20, 2024
90
| NEOGENOMICS, INC. | ||||||||
ITEM 9B. OTHER INFORMATION
None.
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item 10 is incorporated herein by reference to our Definitive Proxy Statement relating to our 2024 Annual Meeting of Stockholders. We intend to file such Definitive Proxy Statement with the SEC pursuant to Regulation 14A within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 will be included in the Definitive Proxy Statement referenced above in Item 10 and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item 12 will be included in the Definitive Proxy Statement referenced above in Item 10 and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
The information required by this Item 13 will be included in the Definitive Proxy Statement referenced above in Item 10 and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this Item 14 will be included in the Definitive Proxy Statement referenced above in Item 10 and is incorporated herein by reference.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) The following documents are filed as part of this Annual Report on Form 10-K:
(i) Financial Statements (included in Item 8 of this Annual Report on Form 10-K):
The following Consolidated Financial Statements of the Company and the Report of Deloitte & Touche LLP, Independent Registered Public Accounting Firm, are included in Part II, Item 8 of this Annual Report on Form 10-K:
1. Report of Independent Registered Public Accounting Firm – Deloitte & Touche LLP
2. Consolidated Balance Sheets as of December 31, 2023 and 2022
3. Consolidated Statements of Operations for the years ended December 31, 2023, 2022 and 2021
4. Consolidated Statements of Comprehensive (Loss) Income for the year ended December 31, 2023, 2022 and 2021
5. Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2023, 2022 and 2021
6. Consolidated Statements of Cash Flows for the years ended December 31, 2023, 2022 and 2021
7. Notes to Consolidated Financial Statements
91
| NEOGENOMICS, INC. | ||||||||
(ii) Financial Statement Schedules.
All schedules are omitted because they are not applicable or the required information is shown in the Consolidated Financial Statements or the Notes thereto.
(iii) Exhibits.
The exhibits required by Item 601 of Regulation S-K are listed in paragraph (b) below.
(b) Exhibits.
The following exhibits are filed herewith or are incorporated by reference to exhibits filed with the SEC:
| Exhibit Number | Description of Exhibit | Location | ||||||||||||
| 3.1 | Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2019 as filed with the SEC on February 28, 2020. |
|||||||||||||
| 3.2 | Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2015, as filed with the SEC on November 6, 2015. |
|||||||||||||
| 4.1 | Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2019 as filed with the SEC on February 28, 2020. |
|||||||||||||
| 4.2 | Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on May 4, 2020. |
|||||||||||||
| 4.3 | Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on May 4, 2020. |
|||||||||||||
| 4.4 | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the SEC on January 11, 2021. | |||||||||||||
| 4.5 | Incorporated by reference to the Company’s Current Report on form 8-K filed with the SEC on January 11, 2021. | |||||||||||||
| 10.3* |
|
Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2016, as filed with the SEC on November 7, 2016. | ||||||||||||
| 10.4* | Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2021, as filed with the SEC on February 25, 2022. | |||||||||||||
| 10.5* | Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, as filed with the SEC on March 15, 2016. | |||||||||||||
| 10.6* | Incorporated by reference to the Company’s Definitive Proxy Statement, dated April 24, 2017, as filed with the SEC on April 25, 2017. | |||||||||||||
92
| NEOGENOMICS, INC. | ||||||||
| 10.7* | Incorporated by reference to Annex A of the Company’s Definitive Proxy Statement on Schedule 14A as filed on April 15, 2021. | |||||||||||||
| 10.8* | Incorporated by reference to Annex A of the Company’s Definitive Proxy Statement on Schedule 14A as filed on April 7, 2023. | |||||||||||||
| 10.9* | Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2023, as filed with the SEC on May 9, 2023. | |||||||||||||
| 10.10* | Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on May 17, 2023. | |||||||||||||
| 10.11* | Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on July 21, 2022. |
|||||||||||||
| 10.12* | Incorporated by reference to the Company’s Registration Statement on Form S-8 as filed with the SEC on August 12, 2022. |
|||||||||||||
| 10.13* | Incorporated by reference to the Company’s Registration Statement on Form S-8 as filed with the SEC on August 12, 2022. |
|||||||||||||
| 10.14* | Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2022, as filed with the SEC on November 8, 2022. | |||||||||||||
| 10.15* | Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the SEC on February 24, 2023. | |||||||||||||
| 10.16* | Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the SEC on February 24, 2023. | |||||||||||||
| 10.17* | Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on May 17, 2023. | |||||||||||||
| 10.18* | Incorporated by reference to the Company’s Registration Statement on Form S-8 as filed with the SEC on December 5, 2022. |
|||||||||||||
| 10.19* | Incorporated by reference to the Company’s Registration Statement on Form S-8 as filed with the SEC on December 5, 2022. |
|||||||||||||
| 10.20* | Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on December 6, 2022. |
|||||||||||||
| 14.1 | Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on July 20, 2011. | |||||||||||||
| 21.1 | Provided herewith. | |||||||||||||
| 23.1 | Provided herewith. | |||||||||||||
| 31.1 | Provided herewith. | |||||||||||||
93
| NEOGENOMICS, INC. | ||||||||
| 31.2 | Provided herewith. | |||||||||||||
| 32.1** | Provided herewith. | |||||||||||||
| 97.1* | Provided herewith. | |||||||||||||
| 101.INS | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | Provided herewith. | ||||||||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document | Provided herewith. | ||||||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | Provided herewith. | ||||||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | Provided herewith. | ||||||||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | Provided herewith. | ||||||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | Provided herewith. | ||||||||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | Provided herewith. | ||||||||||||
| * | Denotes a management contract or compensatory plan or arrangement. | |||||||||||||
| ** | The certification attached as Exhibit 32.1 that accompanies this Form 10-K is not deemed filed with the SEC and is not to be incorporated by reference into any filing of NeoGenomics, Inc. under the Securities Act or the Exchange Act, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing. | |||||||||||||
ITEM 16. FORM 10-K SUMMARY
None.
94
| NEOGENOMICS, INC. | ||||||||
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Date: February 20, 2024 |
NEOGENOMICS, INC. | ||||||||||||||||
| By: | /s/ Christopher M. Smith | ||||||||||||||||
| Name: | Christopher M. Smith | ||||||||||||||||
| Title: | Director and Chief Executive Officer | ||||||||||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
95
| NEOGENOMICS, INC. | ||||||||
| Signatures | Title(s) | Date | ||||||||||||
| /s/ Christopher M. Smith | Director and Chief Executive Officer (Principal Executive Officer) |
February 20, 2024 | ||||||||||||
| Christopher M. Smith | ||||||||||||||
| /s/ Jeffrey S. Sherman | Chief Financial Officer (Principal Financial Officer) |
February 20, 2024 | ||||||||||||
| Jeffrey S. Sherman | ||||||||||||||
| /s/ Gregory D. Aunan | Senior Vice President & Chief Accounting Officer (Principal Accounting Officer) |
February 20, 2024 | ||||||||||||
| Gregory D. Aunan | ||||||||||||||
| /s/ Lynn A. Tetrault | Chair of the Board | February 20, 2024 | ||||||||||||
| Lynn A. Tetrault | ||||||||||||||
| /s/ Bruce K. Crowther | Director | February 20, 2024 | ||||||||||||
| Bruce K. Crowther | ||||||||||||||
| /s/ Elizabeth A. Floegel | Director | February 20, 2024 | ||||||||||||
| Elizabeth A. Floegel | ||||||||||||||
| /s/ Dr. Neil Gunn | Director | February 20, 2024 | ||||||||||||
| Dr. Neil Gunn | ||||||||||||||
| /s/ Dr. Alison L. Hannah | Director | February 20, 2024 | ||||||||||||
| Dr. Alison L. Hannah | ||||||||||||||
| /s/ Stephen M. Kanovsky | Director | February 20, 2024 | ||||||||||||
| Stephen M. Kanovsky | ||||||||||||||
| /s/ Michael A. Kelly | Director | February 20, 2024 | ||||||||||||
| Michael A. Kelly | ||||||||||||||
| /s/ David B. Perez | Director | February 20, 2024 | ||||||||||||
| David B. Perez | ||||||||||||||
| /s/ Anthony P. Zook | Director | February 20, 2024 | ||||||||||||
| Anthony P. Zook | ||||||||||||||
96