EX-99.1
Published on May 9, 2013

MISSION:
To improve patient care through exceptional
cancer genetic diagnostic services
VISION:
By delivering uncompromising quality, exceptional service
and innovative solutions, we are becoming
Americas premier cancer testing laboratory.
VALUES:
Quality Integrity Accountability Teamwork
Employee, Customer & Results Focused
Accelerating Cash Flow & Earnings
| (in thousands, except for tests and percentages) |
FY 2012 | FY 2011 | FY 2010 | |||||||||
| Total Revenue |
$ | 59,867 | $ | 43,484 | $ | 34,371 | ||||||
| % Revenue Growth |
37.7 | % | 26.5 | % | 16.6 | % | ||||||
| Total Gross Profit |
$ | 26,836 | $ | 19,428 | $ | 15,783 | ||||||
| Gross Profit % |
44.8 | % | 44.7 | % | 45.9 | % | ||||||
| Net Income (loss) |
$ | 65 | $ | (1,177 | ) | $ | (3,303 | ) | ||||
| Adjusted EBITDA(1) |
$ | 5,997 | $ | 2,134 | $ | (566 | ) | |||||
| % Growth |
181.0 | % | NA | |||||||||
| Tests |
114,606 | 76,288 | 57,332 | |||||||||
| % Test Growth |
50.2 | % | 33.1 | % | 25.5 | % | ||||||
| (1) | Adjusted EBITDA is defined by NeoGenomics as net income from continuing operations before (i) interest expense, (ii) tax expense, (iii) depreciation and amortization expense, (iv) non-cash stock-based compensation and warrant amortization expense and (v) other extraordinary or non-recurring charges. |

Dear Fellow Shareholders:
From a start-up lab only 10 years ago, NeoGenomics today is a NASDAQ listed, national leader in cancer genetics testing. We have a bold Vision to become Americas premier cancer testing laboratory, and in 2012 we made significant strides in achieving that Vision.
Key 2012 Highlights:
| | Grew revenue by 38% despite the nearly $2.6 million revenue imp act of the TC Grandfather Clause expiration. |
| | Achieved full-year profitability for the first time in our history, and increased earnings before interest, taxes, depreciation, amortization and stock based compensation expenses (Adjusted EBITDA) by 181%. |
| | Developed and launched approximately 50 new testing services to pave the way for future growth. |
| | Built and moved into a new state-of-the-art laboratory facility in Irvine, California and established a Molecular center of excellence and center for R&D. |
| | Strengthened our Management Team, invested in our People, and continued to build a Values-based culture, ending the year with 260 full time employees. |
There are a number of key focus areas for NeoGenomics, and our people are aligned to drive extraordinary success. We made strong progress in each of these areas, and have exciting plans in place.
Growth
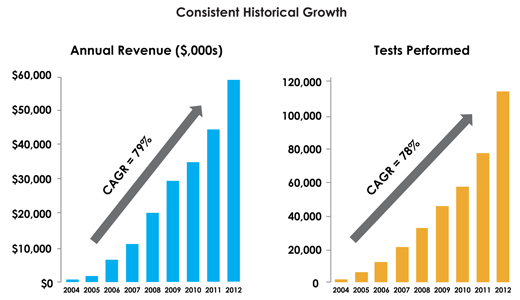
We grew revenue by over $16 million during 2012, and have been told that we are the fastest growing publically traded laboratory in the country. That growth was driven by a combination of important achievements:
| | Our Sales Team made dramatic improvements in productivity to the highest levels in our history. With only 18 Sales Representatives, we generated $16 million of growth by managing the sales process, training, and retaining all of our valuable key sales personnel. |
| NeoGenomics Laboratories. © 2013 All Rights Reserved. | Page 2 |
| | Our measures of client satisfaction were excellent, enabled by our industry-leading levels of turnaround times. This allowed NeoGenomics to retain 96% of our largest clients. |
The foundation of our growth strategy is to establish partnerships with Pathology practices and Hospitals across the country. We are committed to helping these clients grow and prosper in their local markets. We leverage our comprehensive test menus and client-focused Information systems to create a One-Stop-Shop capability and provide for all their cancer genetic testing needs.
Looking forward, we expect to continue to grow at a rate faster than the market. Among our key objectives are:
| | Selectively marketing to Physician Oncology practices, either with our Pathology clients or directly, depending on local market dynamics. |
| | Investing in our Sales and Marketing capabilities by adding experienced Sales professionals to our team. |
| | Realizing significant revenue from new products, by emphasizing the products launched over the last two years in our marketing and commercialization activities. |
Innovation
We took innovation at NeoGenomics to a new level in 2012. Driven by strong Medical and R&D teams, our company made significant accomplishments, which include:
| | Development and launch of 29 new Molecular tests, most of which use the current industry gold standard of bi-directional Sanger Sequencing. |
| | Launch of advanced 10-color Flow Cytometry services on both a global and technical-only basis. |
| | Significant additions to our Immunohistochemistry test menu. |
| | Introduction and launch of a digital image analysis product. |
| | Launch of a new FISH test for ROS1 gene rearrangement for Non-Small Cell Lung cancer. |
| NeoGenomics Laboratories. © 2013 All Rights Reserved. | Page 3 |
| | Introduction of a Neo Array SNP/Cytogentic profile as a molecular karyotype that extends conventional Cytogenetics and FISH testing. |
| | Entry into an exclusive licensing arrangement for use of powerful Support Vector Machine (SVM) pattern recognition technology for cancer testing. |
We believe that relentless innovation is important in this age of revolutionary change in molecular diagnostics. We are continuing to invest in innovation and are beginning to add some proprietary products to our new product pipeline. During 2013, you will see a number of additions to the NeoGenomics test portfolio:
| | A proprietary test for Barretts Esophagus. |
| | An improved and proprietary test for Melanoma. |
| | Next generation sequencing for a variety of cancer types. |
| | Another 20 or more new molecular tests based on new worldwide discoveries. |
Finally, we are continuing our development of a proprietary test for prostate cancer based on plasma and urine. The early results are promising but there is still much work to be done.
Performance
We believe in high-quality and low-cost processes, and we work very hard to continuously improve everything we do at NeoGenomics. To accomplish this, we are making significant investments in quality management, process management, lab automation, information technology, and in our people.
In 2012, we executed initiatives to drive substantial improvements in productivity and lower our cost structure, which enabled us to:
| | Reduce average cost per test by 8.6% to the lowest level in our history. |
| | Increase the number of tests processed per lab employee by 18% to its highest level in our history. |
Over the past three years, we have made tremendous progress in increasing our productivity.
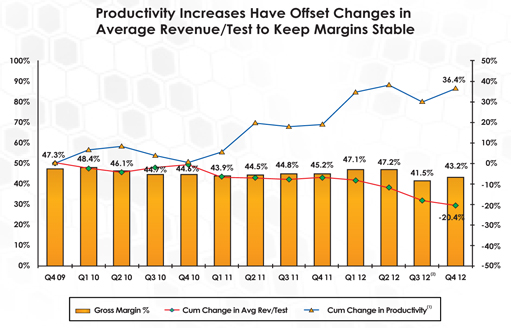
| (1) | Productivity calculated as the average number of lab tests completed per month per laboratory FTE. |
| (2) | The Medicare Technical Component Grandfather expiration took effect on 7/1/12, which resulted in a 7.3% sequential decrease in Avg. Unit Price |
| NeoGenomics Laboratories. © 2013 All Rights Reserved. | Page 4 |
Our best practice teams continue to work on improving our processes. We are implementing Lean Process initiatives, bar coding and scanning technology, new and improved instrumentation to further automate our laboratories, and new IT enhancements that will help us process work more effectively and efficiently.
Industry Dynamics and Economic Environment
As many of you know, in 2012 we encountered a significant challenge. On July 1st of 2012, legislation went into effect that changed a decades old billing practice. Referred to as the expiration of the TC-Grandfather Clause, the change resulted in us having to bill Hospitals for many tests that were previously billed to Medicare directly. The change reduced revenue and profit by approximately $2.6 million during the second half of the year.
NeoGenomics took a leadership role in communicating the change and helping our clients understand and get through the transition. Unfortunately, the change also caused distraction to our sales force and took them away from their important role of growing our company. However, even in the face of this enormous regulatory challenge, we continued to grow and reported our first full year of profitability in our history.
Looking ahead, we expect governmental pressure to continue and to present the entire laboratory industry with reimbursement challenges. In this challenging macroeconomic environment, we continue to take decisive actions to mitigate headwinds and prepare for future growth. We are trying to do our part, as good corporate citizens, to usher in the era and promise of personalized medicine, and to help improve health care in America.
In summary, we are making good progress building and developing our company. Our key laboratory disciplines in Cytogenetics, FISH, Flow Cytometry, IHC and Molecular testing are among the very best in America. Our investments in R&D are positioning us to drive growth in future periods. Our quality and service levels are strong. And our people are dedicated and committed to living our Values and achieving our Vision.
We are very excited about the future for NeoGenomics.
Thank you for your support!
Best regards,
Douglas M. VanOort
Chairman and Chief Executive Officer
| NeoGenomics Laboratories. © 2013 All Rights Reserved. | Page 5 |