EX-99.1
Published on December 23, 2015

Special Shareholder Meeting December 21, 2015 Acquisition of Exhibit 99.1 1

Forward-looking Statements This presentation contains statements which constitute forward-looking statements within the meaning of Section 27A of the Securities Act, as amended; Section 21E of the Securities Exchange Act of 1934; and the Private Securities Litigation Reform Act of 1995. The words “may”, “would”, “could”, “will”, “expect”, “estimate”, “anticipate”, “believe”, “intend”, “plan”, “goal”, and similar expressions and variations thereof are intended to specifically identify forward-looking statements. All statements that are not statements of historical fact are forward-looking statements. Investors and prospective investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and that actual results may differ materially from those projected in the forward-looking statements as a result of various factors. The risks that might cause such differences are identified in our filings with the Securities and Exchange Commission. We undertake no obligation to publicly update or revise the forward looking statements made in this presentation to reflect events or circumstances after the date of this presentation or to reflect the occurrence of unanticipated events. We are soliciting the required approval of our stockholders in connection with our planned acquisition of Clarient by means of a proxy statement, which stockholders should review as it contains important information that stockholders should consider, as well as information about the participants in the solicitation. Stockholders will also be able to obtain the proxy statement, as well as other NeoGenomics filings, without charge, at the SEC’s web site, or from our website at www.neogenomics.com or by writing NeoGenomics’ Corporate Secretary at 12701 Commonwealth Drive, Suite #5, Fort Myers, FL 33913. We and our executive officers and directors may be deemed to be participants in the solicitation of proxies from our stockholders with respect to the proposed Clarient transaction. Information regarding any interests that our executive officers and directors may have in the transaction will be set forth in the proxy statement.
Investment Highlights Fast growing cancer genetics lab servicing Oncologists, Pathologists and Hospitals Strategic client partnerships created by “Tech-Only” model Dynamic, rapidly- growing and consolidating industry Industry-leading revenue & test volume growth Most Comprehensive Cancer Testing Menu in Industry Strong Management Team with large cap lab experience Pending Acquisition of Clarient Should more than Double the Revenue of NeoGenomics in 2016

Experienced Management Team Douglas VanOort, Chairman & CEO Chief Operating Officer, Quest Diagnostics Executive Vice President, Corning Life Sciences, Inc. Maher Albitar, M.D., Chief Medical Officer & Director of R&D Med. Dir. for Hematopathology & Oncology and Chief of R&D, Quest Nichols Institute; Director of Leukemia and Molecular Laboratory, MD Anderson Cancer Center Robert Shovlin, Chief Operating Officer Chief Development Officer, Bostwick Laboratories President & Chief Executive Officer, Aureon Biosciences Steven Jones, Director, EVP – Finance, & Chief Compliance Officer Chairman, Aspen Capital Group; Managing Member, Medical Venture Partners Vice President, Merrill Lynch Investment Banking George Cardoza, Chief Financial Officer CFO, Protocol Global Solutions; Controller, Central Region, Quest Diagnostics Robert Horel, Vice President & General Manager, PathLogic Regional Mgr., US Labs; Product Specialist, Ventana Medical Systems; Pilot, U.S. Navy Steven Ross, Chief Information Officer Vice President Technology, Chico’s FAS, Inc.
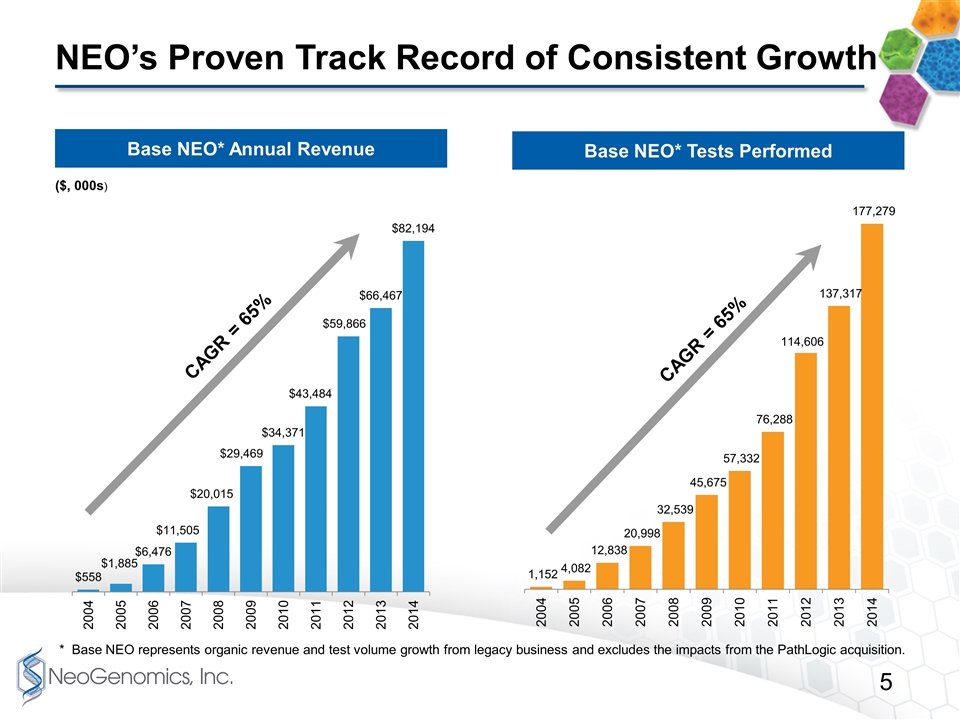
NEO’s Proven Track Record of Consistent Growth Base NEO* Annual Revenue Base NEO* Tests Performed ($, 000s) CAGR = 65% CAGR = 65% * Base NEO represents organic revenue and test volume growth from legacy business and excludes the impacts from the PathLogic acquisition.
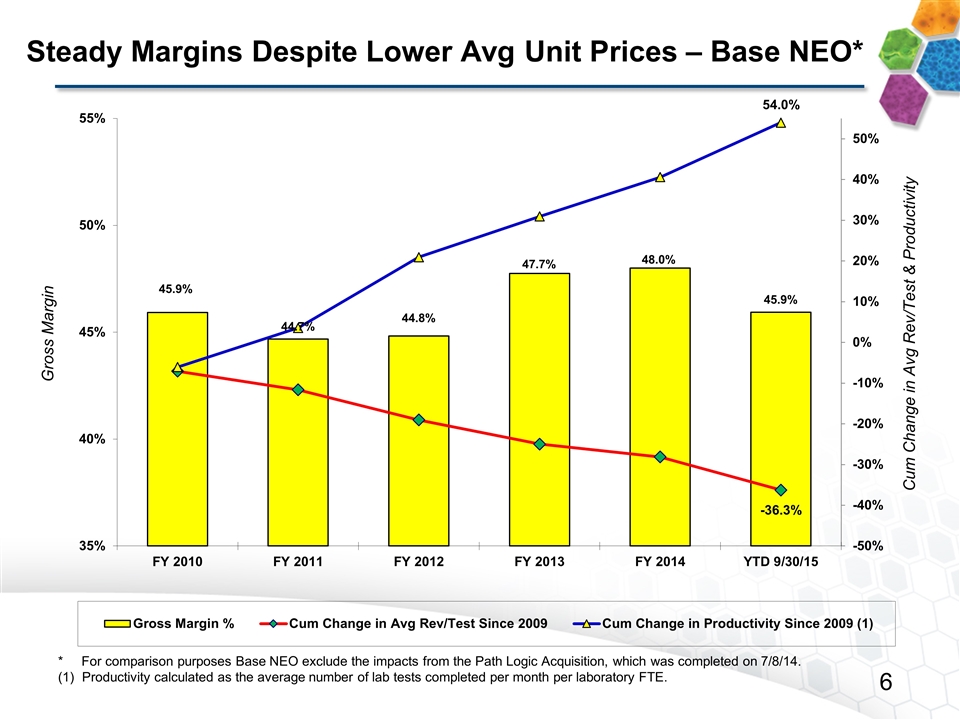
Steady Margins Despite Lower Avg Unit Prices – Base NEO* * For comparison purposes Base NEO exclude the impacts from the Path Logic Acquisition, which was completed on 7/8/14. Productivity calculated as the average number of lab tests completed per month per laboratory FTE.

Customer Targets Pathologists & Hospital Pathology Groups (about 82% of YTD 2015 Revenue ) Enable community Pathologists to practice using sophisticated tools and tests Innovative technical component (TC or “tech-only”) services – Flow, FISH, IHC Outstanding Web-based Lab System & extensive training programs Oncologists & Clinician Groups (about 15% of YTD 2015 Revenue ) Includes Hematologists, Oncologists, Dermatologists, Urologists Disease Panels and comprehensive molecular menus Increasing Opportunity to service larger practices with Tech-only model Clinical Trials & Other (about 3% of YTD 2015 Revenue ) Contract research/clinical trial support work for Pharma clients
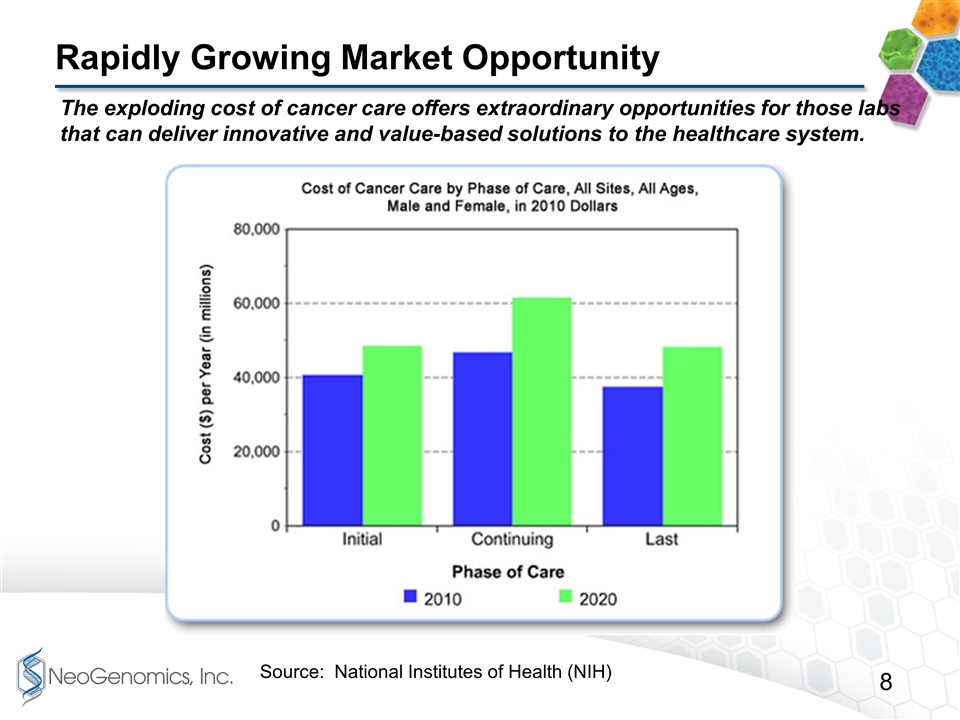
Rapidly Growing Market Opportunity The exploding cost of cancer care offers extraordinary opportunities for those labs that can deliver innovative and value-based solutions to the healthcare system. Source: National Institutes of Health (NIH)
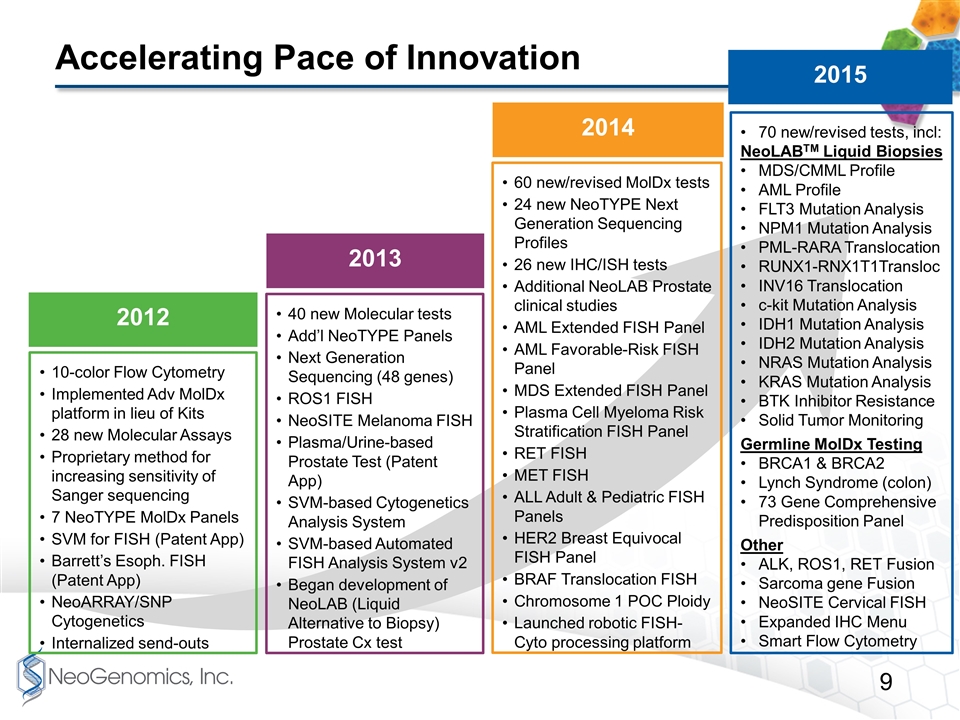
Accelerating Pace of Innovation 70 new/revised tests, incl: NeoLABTM Liquid Biopsies MDS/CMML Profile AML Profile FLT3 Mutation Analysis NPM1 Mutation Analysis PML-RARA Translocation RUNX1-RNX1T1Transloc INV16 Translocation c-kit Mutation Analysis IDH1 Mutation Analysis IDH2 Mutation Analysis NRAS Mutation Analysis KRAS Mutation Analysis BTK Inhibitor Resistance Solid Tumor Monitoring Germline MolDx Testing BRCA1 & BRCA2 Lynch Syndrome (colon) 73 Gene Comprehensive Predisposition Panel Other ALK, ROS1, RET Fusion Sarcoma gene Fusion NeoSITE Cervical FISH Expanded IHC Menu Smart Flow Cytometry 10-color Flow Cytometry Implemented Adv MolDx platform in lieu of Kits 28 new Molecular Assays Proprietary method for increasing sensitivity of Sanger sequencing 7 NeoTYPE MolDx Panels SVM for FISH (Patent App) Barrett’s Esoph. FISH (Patent App) NeoARRAY/SNP Cytogenetics Internalized send-outs 40 new Molecular tests Add’l NeoTYPE Panels Next Generation Sequencing (48 genes) ROS1 FISH NeoSITE Melanoma FISH Plasma/Urine-based Prostate Test (Patent App) SVM-based Cytogenetics Analysis System SVM-based Automated FISH Analysis System v2 Began development of NeoLAB (Liquid Alternative to Biopsy) Prostate Cx test 2015 2012 2013 60 new/revised MolDx tests 24 new NeoTYPE Next Generation Sequencing Profiles 26 new IHC/ISH tests Additional NeoLAB Prostate clinical studies AML Extended FISH Panel AML Favorable-Risk FISH Panel MDS Extended FISH Panel Plasma Cell Myeloma Risk Stratification FISH Panel RET FISH MET FISH ALL Adult & Pediatric FISH Panels HER2 Breast Equivocal FISH Panel BRAF Translocation FISH Chromosome 1 POC Ploidy Launched robotic FISH-Cyto processing platform 2014
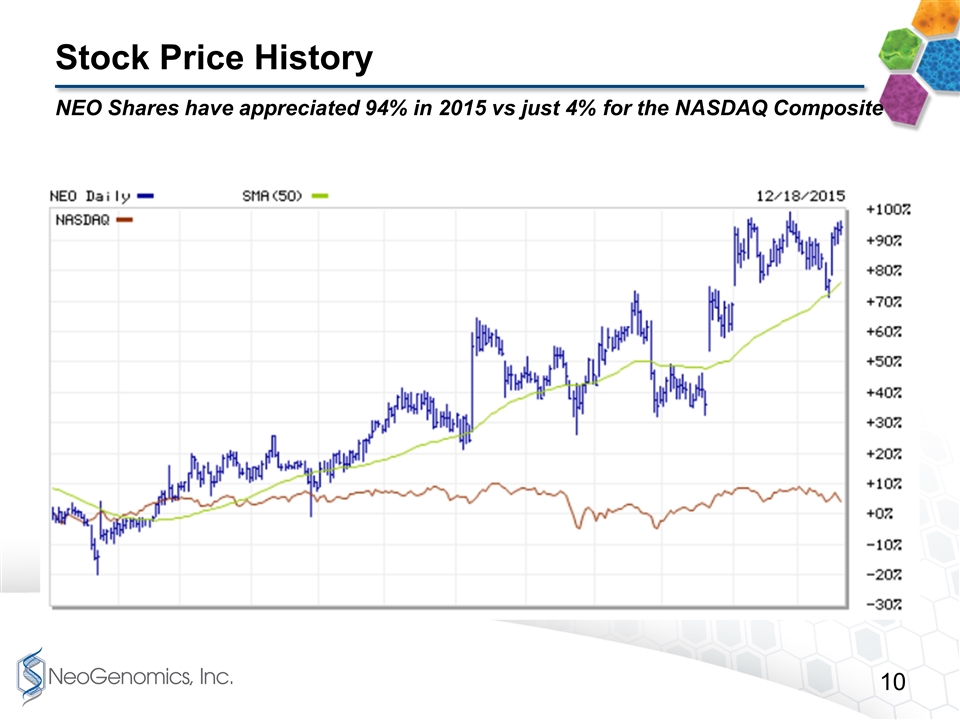
Stock Price History NEO Shares have appreciated 94% in 2015 vs just 4% for the NASDAQ Composite
Clarient Transaction Background NeoGenomics’ has had a long-standing Vision to become the leading cancer testing and Information company Key Strategic Elements of Strategy High-quality and low-cost provider. Growth by market share gains as “one-stop-shop” for cancer genetic testing. Innovate aggressively to advance precision medicine Diversify product line and enter BioPharma market. Communicated desire to accelerate achievement of Vision and Strategies through M&A. Clarient is a transformational acquisition for NEO – creates ability to transform NEO quickly into industry leader. We were in a strategic dialogue with GE for a year, and are extremely pleased with the outcome.
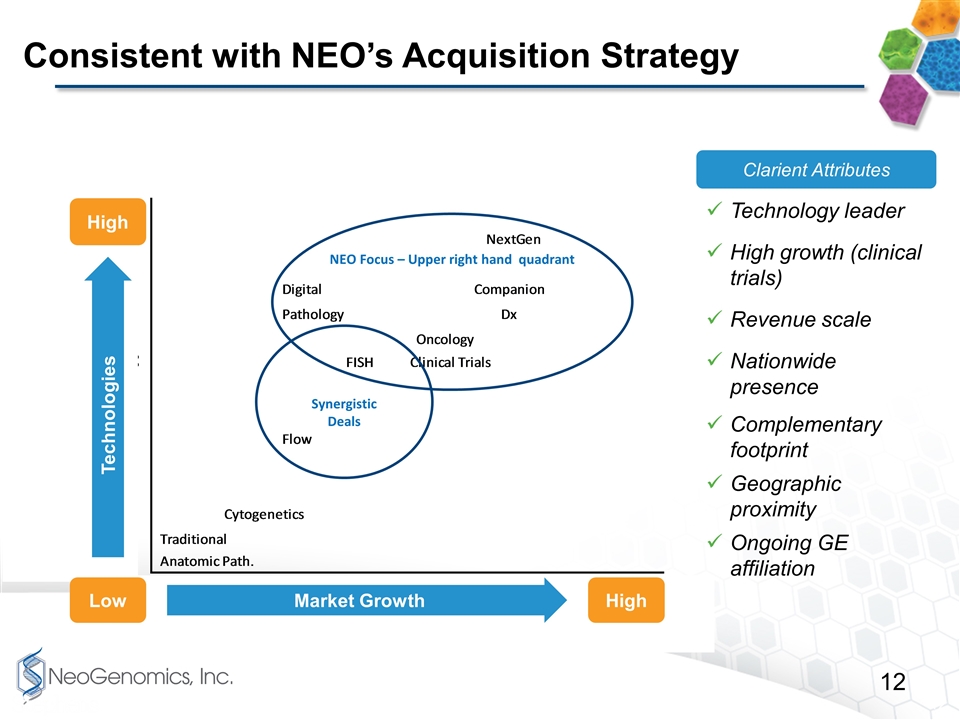
Consistent with NEO’s Acquisition Strategy NEO Focus – Upper right hand quadrant Synergistic Deals High High Low Market Growth Technologies Clarient Attributes Technology leader High growth (clinical trials) Revenue scale Nationwide presence Complementary footprint Geographic proximity Ongoing GE affiliation
Strategic Rationale Lab scale allows opportunity to be the low-cost provider in each key laboratory discipline. Better balance of clinical products to reduce concentration risk. Significantly enhanced Clinical Trials capability. Create greater economies of scale in IT, Regulatory, Billing, Compliance, etc. Leverages and optimizes the Sales and Marketing function across a broader revenue base with more diverse geographic coverage. Leverages innovation & product development across broader client base. Greater ability to serve Managed Care and large buyers in a significant way. Better “one-stop-shop” capability and services to existing and new clients.
Key Benefits Synergy potential of $20mm - $30mm/year within 3-5 year Laboratory, Purchasing, Cross-selling, etc. East Coast/West Coast labs Combine Irvine and Aliso Viejo Low cost position in every testing discipline FISH, Flow, Cytogenetics, IHC, Digital Pathology, Molecular Leadership in hematological and solid tumor cancers One-stop-shop for clients with broad geographical coverage Significant clinical trials business Combined business approximates $25mm revenue Potential to be a leading consolidator going forward GE as significant long-term Investor Collaboration in Bioinformatics in Precision Oncology

Clinical Trials - BioPharma A rapidly growing part of Clarient’s business and a fantastic opportunity . . . Over $20mm/yr. in current business Strong relationships with several leading Pharmaceutical firms PDL1 22C3 – FDA Approved companion diagnostic for Keytruda Continued push into Companion Dx and personalized medicine Approved vendor with top firms On several firms advisory boards Solid pipeline and future growth opportunities

Revenue Synergies – Cross Selling - World Class Molecular Testing Menu - NeoTYPE Panels - Extensive Tech-only Service Offering - Excellence in Heme Laboratory Testing - Leading growth-oriented Sales & Mkting Team - Industry Leading Digital Pathology Platform - Strong capabilities in IHC/Histology - Leading Solid Tumor Laboratory We plan to bring the best of both Companies to our existing clients…
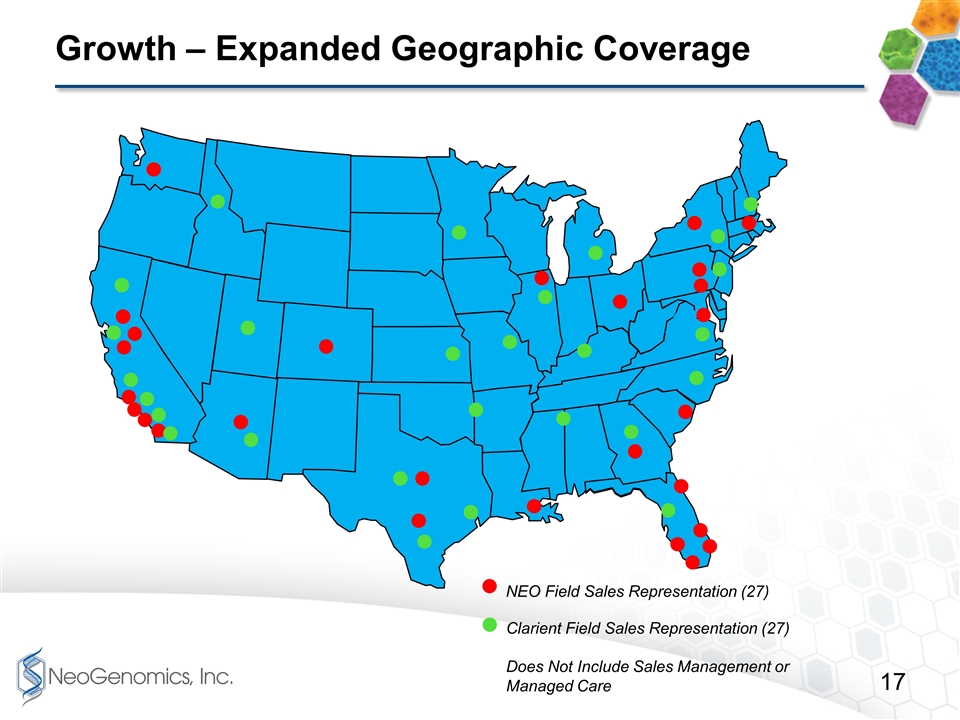
Growth – Expanded Geographic Coverage NEO Field Sales Representation (27) Clarient Field Sales Representation (27) Does Not Include Sales Management or Managed Care
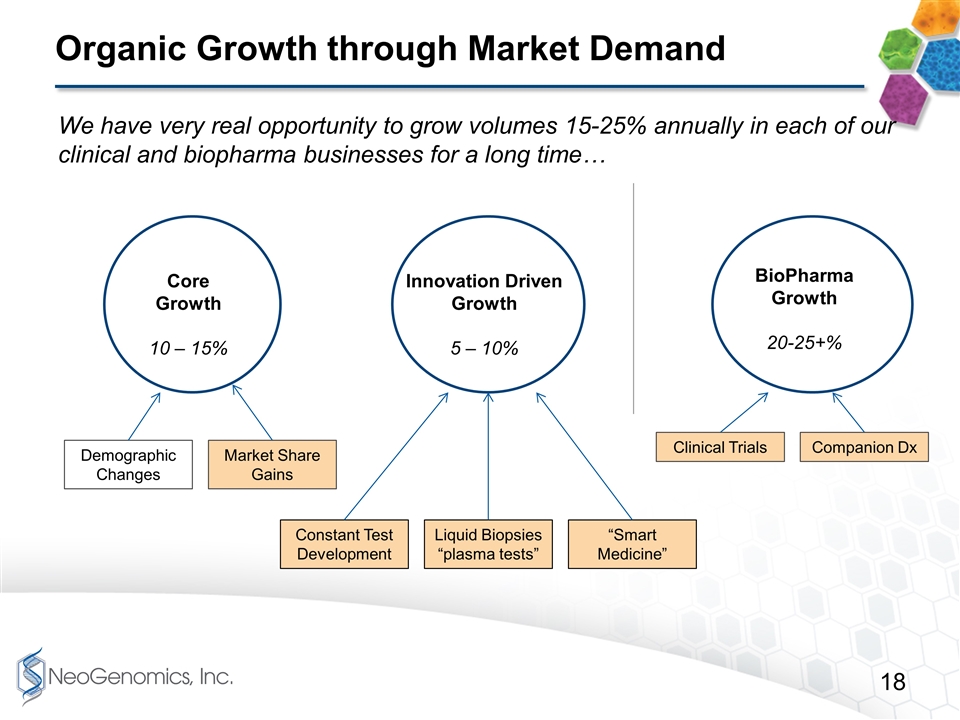
Organic Growth through Market Demand Core Growth 10 – 15% Demographic Changes Market Share Gains Innovation Driven Growth 5 – 10% Constant Test Development Liquid Biopsies “plasma tests” “Smart Medicine” BioPharma Growth 20-25+% Clinical Trials Companion Dx We have very real opportunity to grow volumes 15-25% annually in each of our clinical and biopharma businesses for a long time…
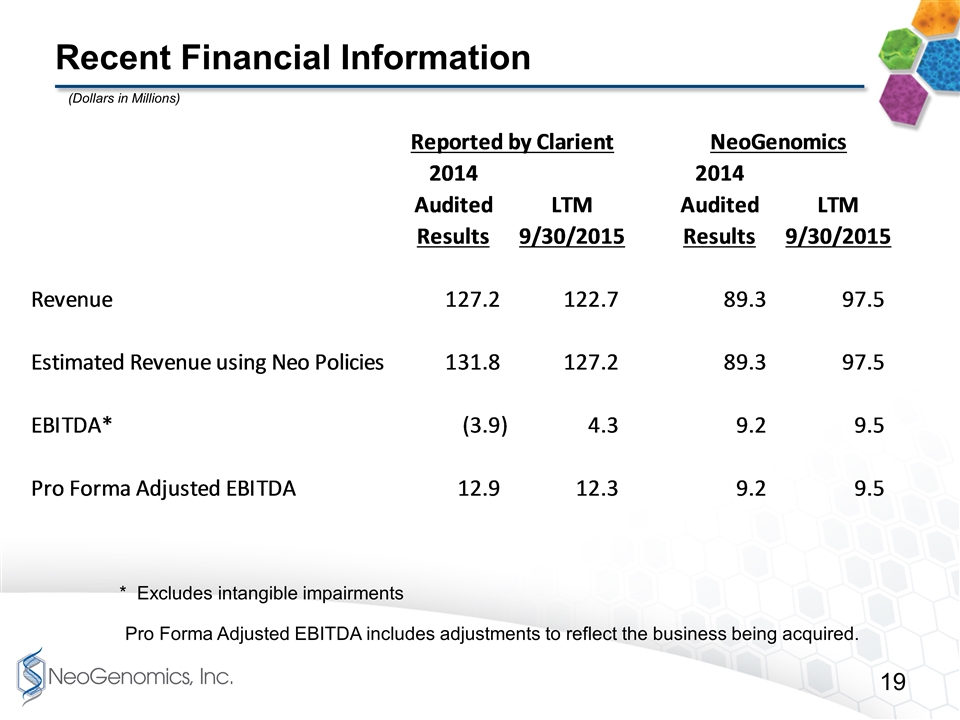
Recent Financial Information * Excludes intangible impairments Pro Forma Adjusted EBITDA includes adjustments to reflect the business being acquired. (Dollars in Millions)
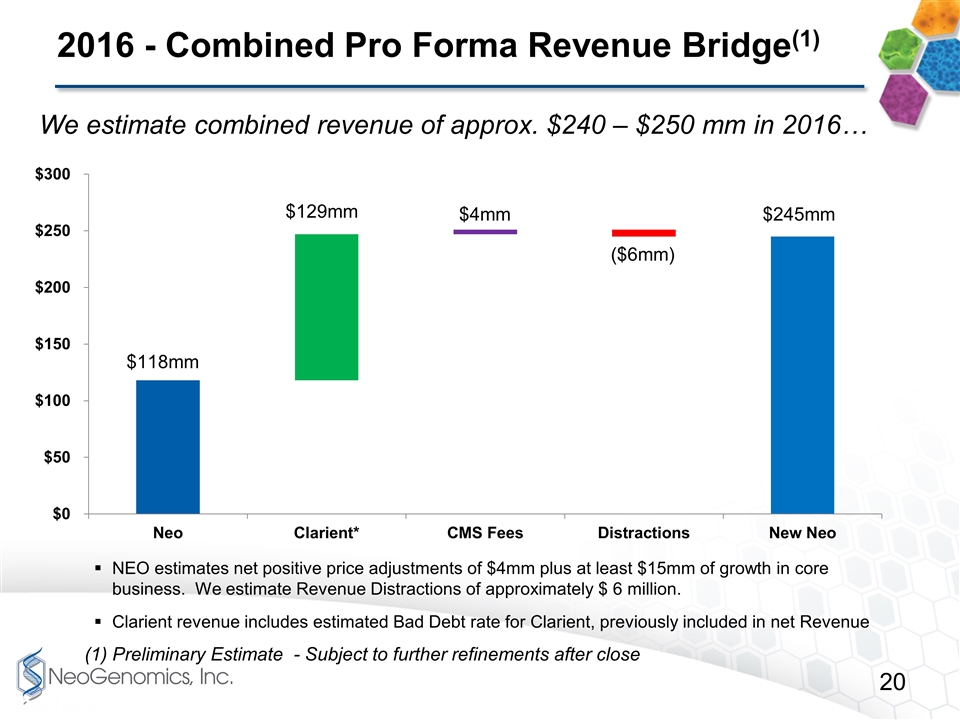
2016 - Combined Pro Forma Revenue Bridge(1) (1) Preliminary Estimate - Subject to further refinements after close We estimate combined revenue of approx. $240 – $250 mm in 2016… NEO estimates net positive price adjustments of $4mm plus at least $15mm of growth in core business. We estimate Revenue Distractions of approximately $ 6 million. Clarient revenue includes estimated Bad Debt rate for Clarient, previously included in net Revenue $4mm $245mm
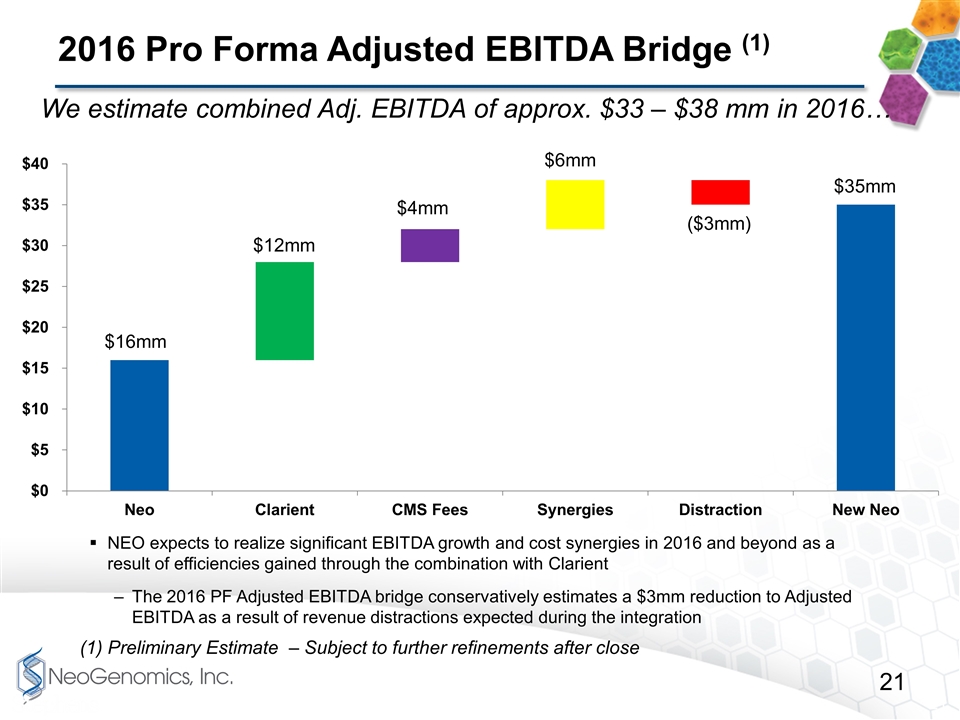
2016 Pro Forma Adjusted EBITDA Bridge (1) NEO expects to realize significant EBITDA growth and cost synergies in 2016 and beyond as a result of efficiencies gained through the combination with Clarient The 2016 PF Adjusted EBITDA bridge conservatively estimates a $3mm reduction to Adjusted EBITDA as a result of revenue distractions expected during the integration (1) Preliminary Estimate – Subject to further refinements after close We estimate combined Adj. EBITDA of approx. $33 – $38 mm in 2016…
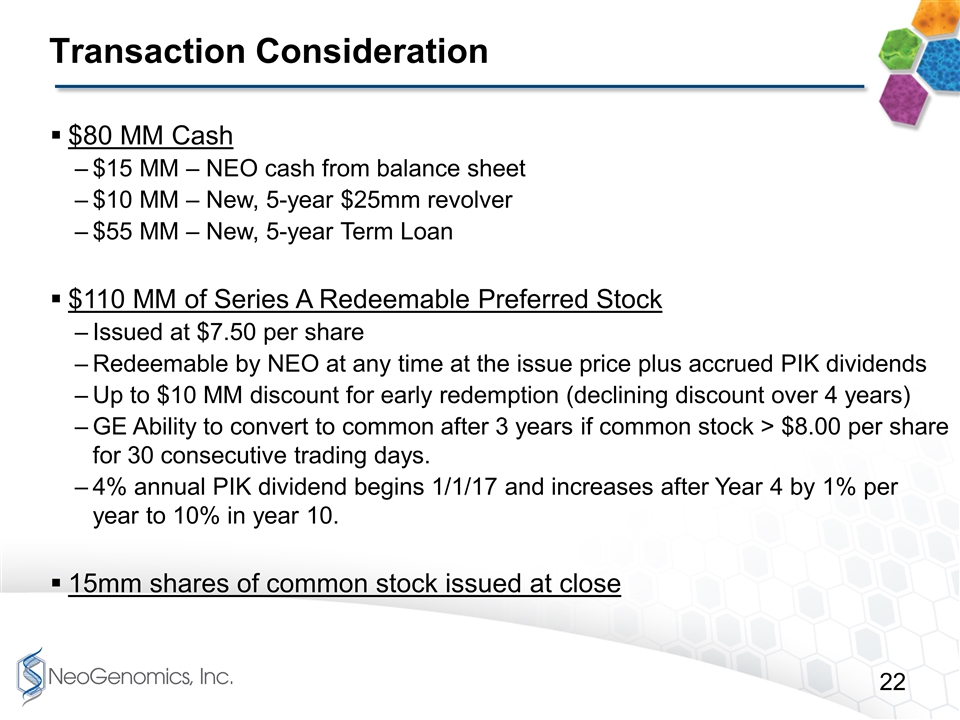
Transaction Consideration $80 MM Cash $15 MM – NEO cash from balance sheet $10 MM – New, 5-year $25mm revolver $55 MM – New, 5-year Term Loan $110 MM of Series A Redeemable Preferred Stock Issued at $7.50 per share Redeemable by NEO at any time at the issue price plus accrued PIK dividends Up to $10 MM discount for early redemption (declining discount over 4 years) GE Ability to convert to common after 3 years if common stock > $8.00 per share for 30 consecutive trading days. 4% annual PIK dividend begins 1/1/17 and increases after Year 4 by 1% per year to 10% in year 10. 15mm shares of common stock issued at close
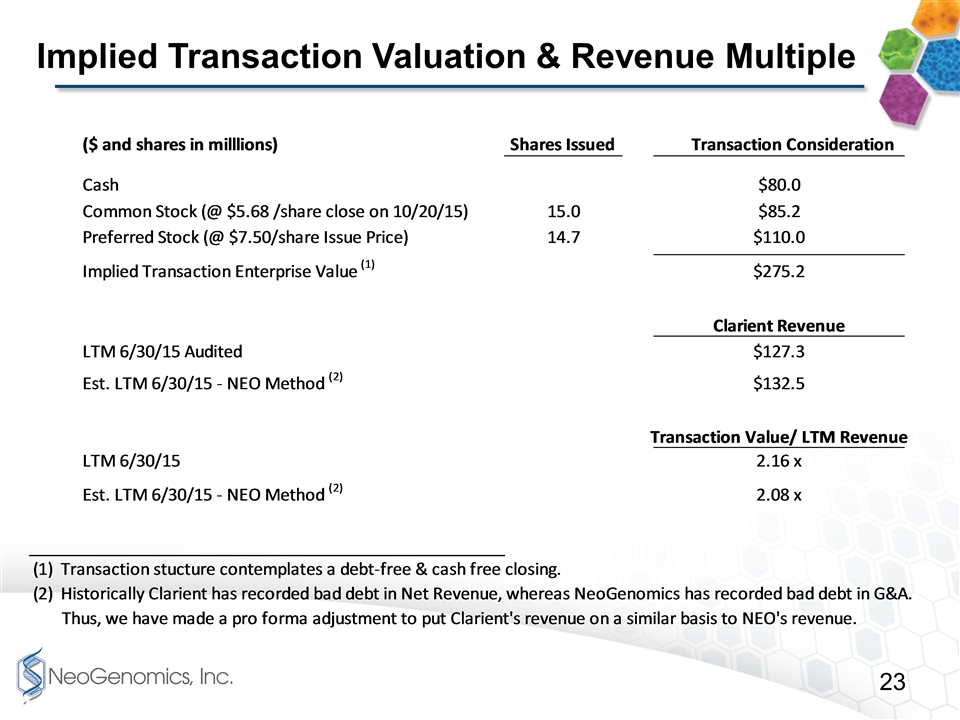
Implied Transaction Valuation & Revenue Multiple
NeoGenomics Vision and Goals Leadership Goals: To be the leading oncology-focused testing and information company in the World. Competencies: Outstanding Scientific, Medical and Informatics expertise. Partnerships for efficiency/effectiveness across health-care care continuum Disciplined process management and quality systems. Entrepreneurial, values-driven culture. Financial Performance Goals: Consistent and sustainable double-digit revenue growth. Clinical Trials > 15% of revenue. Adjusted EBITDA margin of 20-25% when annual revenue >$300 MM.

Matters To Be Voted Upon at Shareholder’s Meeting Issue 15 MM shares of Common Stock and 14.667 MM shares of Series A Preferred Stock, as may be adjusted, to GE Medical Holdings AB Increase authorized shares of Common Stock to 250 MM Increase authorized share of preferred stock to 50 MM Approval of the Purchase Agreement and the Transaction Amend the NEO Equity Incentive Plan to increase the shares authorized for issuance by 3 MM shares to 12.5 MM shares total Authority to adjourn the special meeting, if necessary, to solicit additional votes and proxies if there are insufficient votes at the time of the special meeting

Appendix 20 Appendix
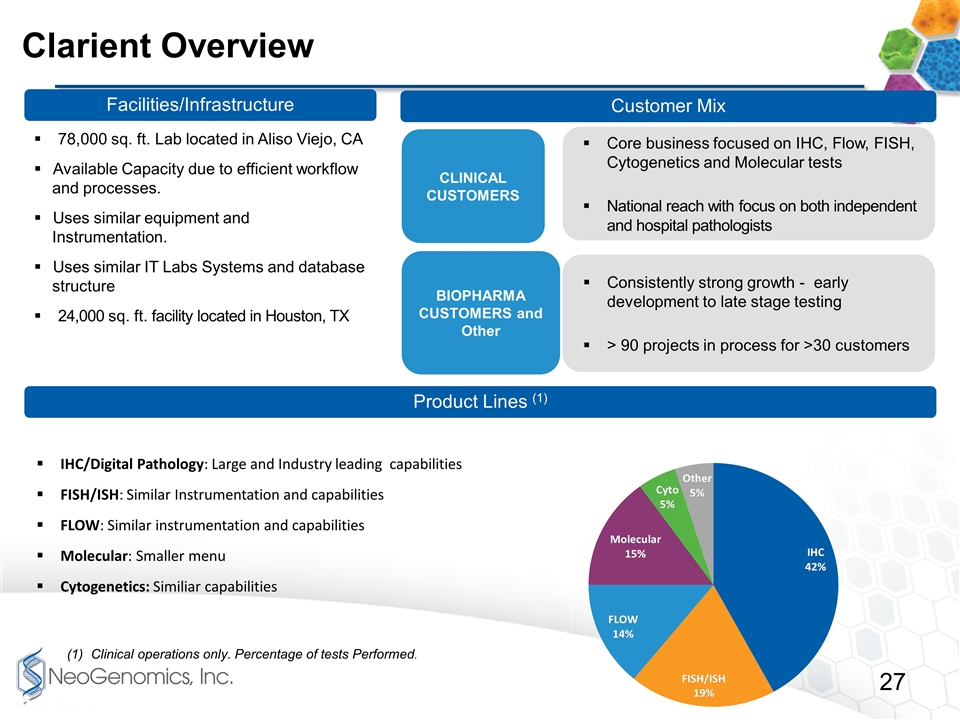
Core business focused on IHC, Flow, FISH, Cytogenetics and Molecular tests National reach with focus on both independent and hospital pathologists Consistently strong growth - early development to late stage testing > 90 projects in process for >30 customers Clarient Overview IHC/Digital Pathology: Large and Industry leading capabilities FISH/ISH: Similar Instrumentation and capabilities FLOW: Similar instrumentation and capabilities Molecular: Smaller menu Cytogenetics: Similiar capabilities Facilities/Infrastructure Customer Mix Product Lines (1) 78,000 sq. ft. Lab located in Aliso Viejo, CA Available Capacity due to efficient workflow and processes. Uses similar equipment and Instrumentation. Uses similar IT Labs Systems and database structure 24,000 sq. ft. facility located in Houston, TX Clinical operations only. Percentage of tests Performed. CLINICAL CUSTOMERS BIOPHARMA CUSTOMERS and Other
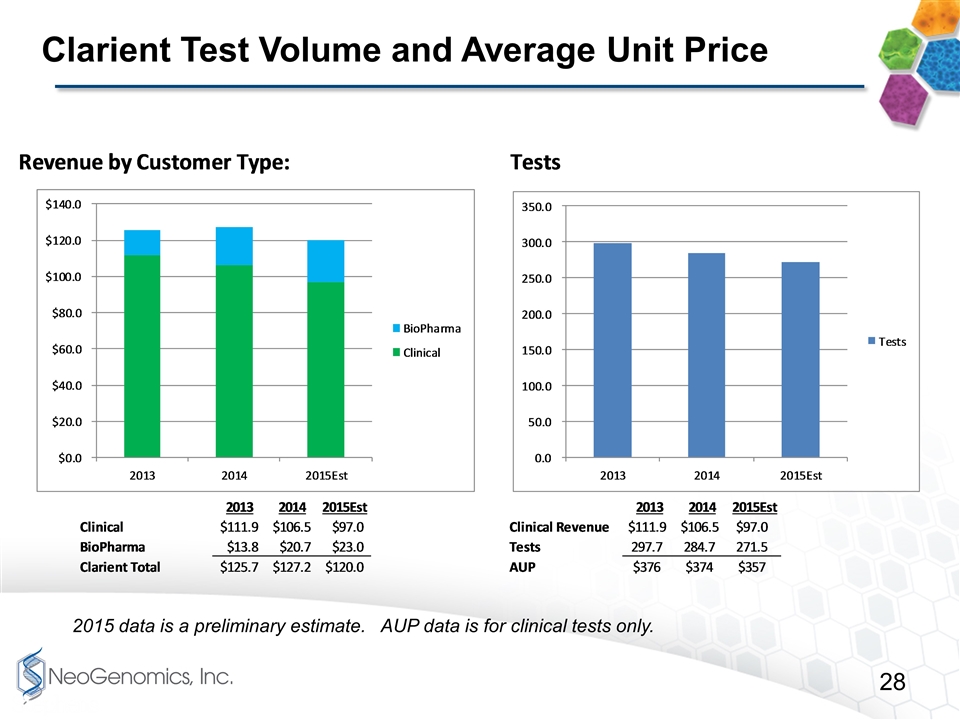
Clarient Test Volume and Average Unit Price 2015 data is a preliminary estimate. AUP data is for clinical tests only.
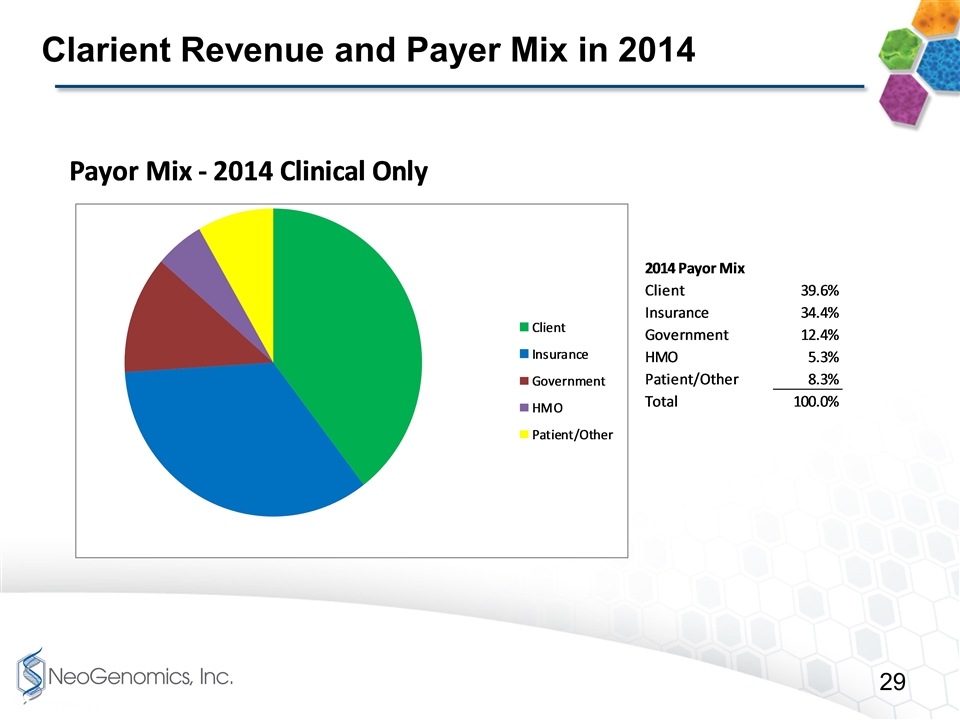
Clarient Revenue and Payer Mix in 2014