EX-99.1
Published on June 8, 2016

Annual Shareholder Meeting June 7, 2016 NASDAQ: NEO Exhibit 99.1

Forward-looking Statements This presentation contains statements which constitute forward-looking statements within the meaning of Section 27A of the Securities Act, as amended; Section 21E of the Securities Exchange Act of 1934; and the Private Securities Litigation Reform Act of 1995. The words “may”, “would”, “could”, “will”, “expect”, “estimate”, “anticipate”, “believe”, “intend”, “plan”, “goal”, and similar expressions and variations thereof are intended to specifically identify forward-looking statements. All statements that are not statements of historical fact are forward-looking statements. Investors and prospective investors are cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and that actual results may differ materially from those projected in the forward-looking statements as a result of various factors. The risks that might cause such differences are identified in our filings with the Securities and Exchange Commission. We undertake no obligation to publicly update or revise the forward looking statements made in this presentation to reflect events or circumstances after the date of this presentation or to reflect the occurrence of unanticipated events.

Board of Directors Douglas M. VanOort, Chairman of Board Chairman since 2009. Significant prior industry and executive experience. Bruce K. Crowther, Chairman of Compliance Committee Director since 2014. Retired as Chairman & CEO of Northwest Community Healthcare. Alison L. Hannah, M.D. Director since 2015. Consultant in field of Oncology Drug Development. Raymond R. Hipp, Chairman of Audit Committee Director since 2011. Retired CEO with significant finance and M&A experience. Kevin C. Johnson Director since 2010. Former CEO of Dianon Systems Steven C. Jones Director and NEO Executive since 2003. Significant financial and executive experience. Kieran P. Murphy Director since 2016. CEO, GE Healthcare Life Sciences William J. Robison, Chairman of Nominating and Governance Committee Director since 2007. Retired as Executive Vice President, Pfizer, Inc. Lynn A. Tetrault, Chairman of Compensation Committee Director since 2015. Retired as Executive Vice President, AstraZeneca PLC

Experienced Management Team Douglas VanOort, Chairman & CEO Chief Operating Officer, Quest Diagnostics Executive Vice President, Corning Life Sciences, Inc. Maher Albitar, M.D., Chief Medical Officer & Director of R&D Med. Dir. for Hematopathology & Oncology and Chief of R&D, Quest Nichols Institute; Director of Leukemia and Molecular Laboratory, MD Anderson Cancer Center Robert Shovlin, Chief Growth Officer Chief Development Officer, Bostwick Laboratories; President & Chief Executive Officer, Aureon Biosciences Steven Jones, Director, EVP – Finance, & Chief Compliance Officer Chairman, Aspen Capital Group; Vice President, Merrill Lynch Investment Banking George Cardoza, Chief Financial Officer CFO, Protocol Global Solutions; Controller, Central Region, Quest Diagnostics Steven Brodie – Chief Scientific Officer Senior Director - Quest Diagnostics Steven Ross, Chief Information Officer Vice President Technology, Chico’s FAS, Inc. Mark Machulcz, Vice President, Operations Director of Operations - Clarient Diagnostic Services

2015 Highlights Increased test volume by 25% in our base business Reduced cost-per-test in our base business by 9% Launched over 70 new or enhanced tests Completed the acquisition of Clarient, Inc. from GE for approximately $250 million Positioned the company as an emerging leader.
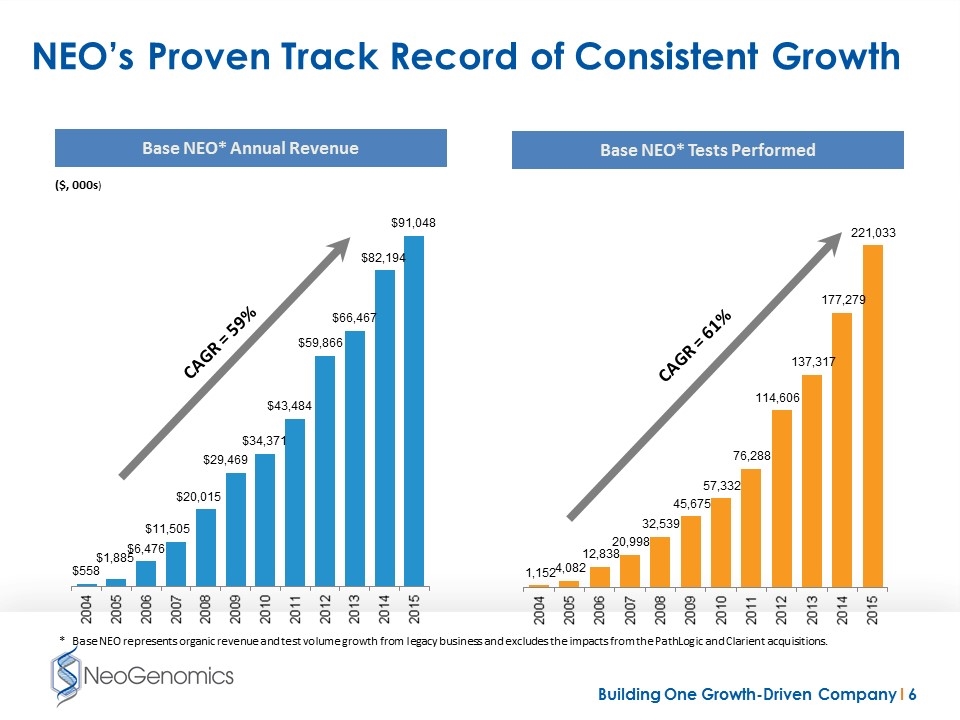
NEO’s Proven Track Record of Consistent Growth Base NEO* Annual Revenue Base NEO* Tests Performed ($, 000s) CAGR = 59% CAGR = 61% * Base NEO represents organic revenue and test volume growth from legacy business and excludes the impacts from the PathLogic and Clarient acquisitions.
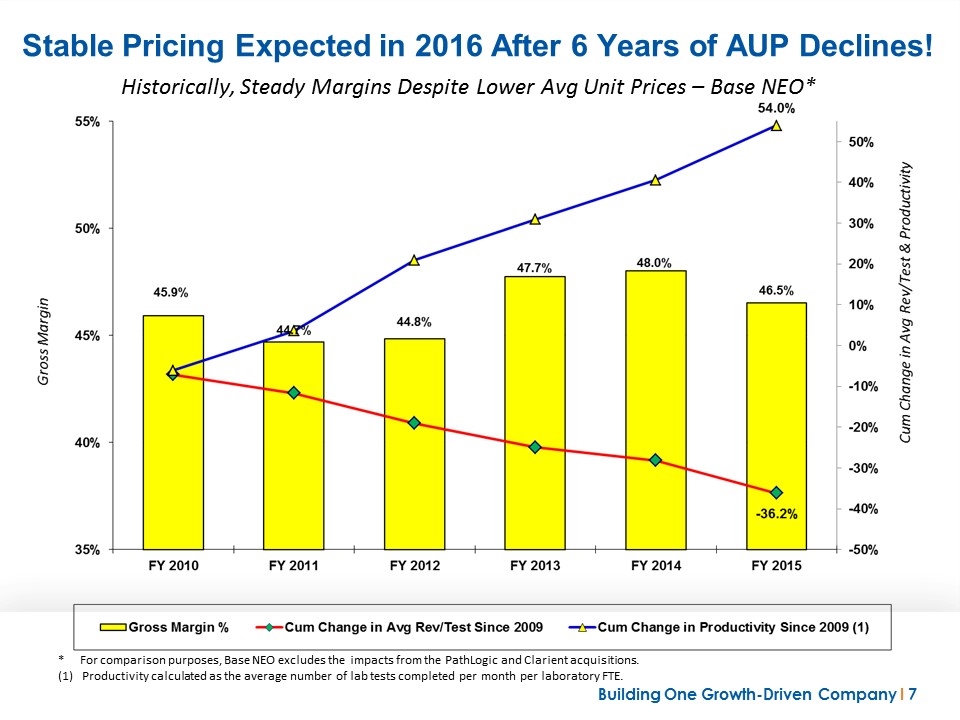
Stable Pricing Expected in 2016 After 6 Years of AUP Declines! * For comparison purposes, Base NEO excludes the impacts from the PathLogic and Clarient acquisitions. Productivity calculated as the average number of lab tests completed per month per laboratory FTE. Historically, Steady Margins Despite Lower Avg Unit Prices – Base NEO*

Customer Targets Pathologists & Hospital Pathology Groups (about 81% of 2015 Revenue*) Enable community Pathologists to practice using sophisticated tools and tests Innovative technical component (TC or “tech-only”) services – Flow, FISH, IHC Outstanding Web-based Lab System & extensive training programs Oncologists & Clinician Groups (about 15% of 2015 Revenue*) Includes Hematologists, Oncologists, Dermatologists, Urologists Disease Panels and comprehensive molecular menus Increasing Opportunity to service larger practices with Tech-only model Clinical Trials & Other (about 4% of 2015 Revenue*) Contract research/clinical trial support work for Pharma clients * Percentages are for Base NEO only and exclude the impacts from the PathLogic and Clarient acquisitions.
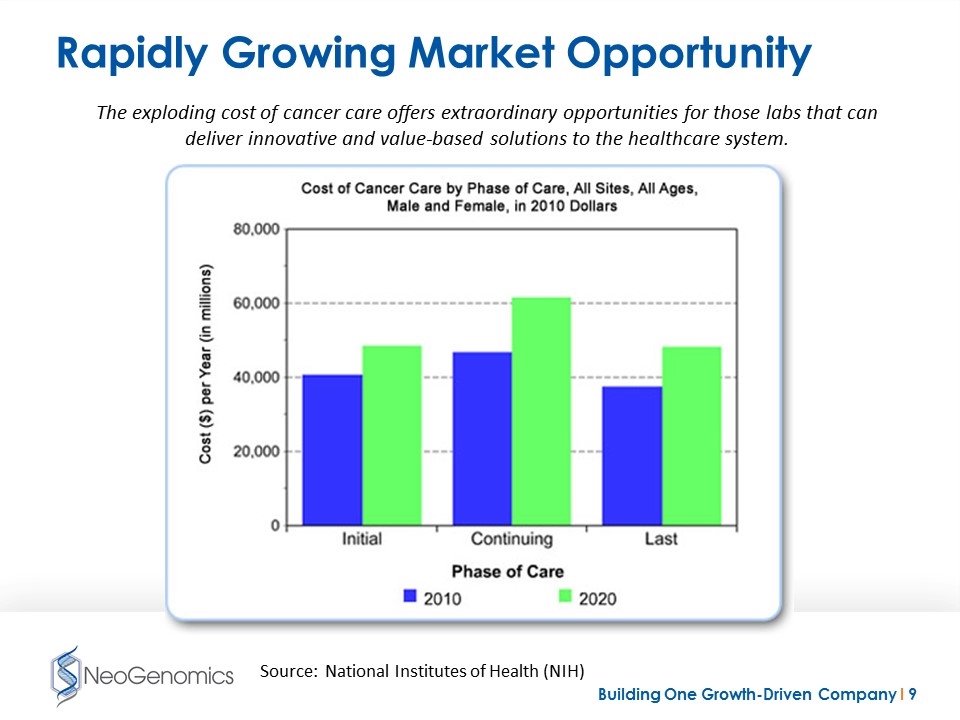
Rapidly Growing Market Opportunity The exploding cost of cancer care offers extraordinary opportunities for those labs that can deliver innovative and value-based solutions to the healthcare system. Source: National Institutes of Health (NIH)
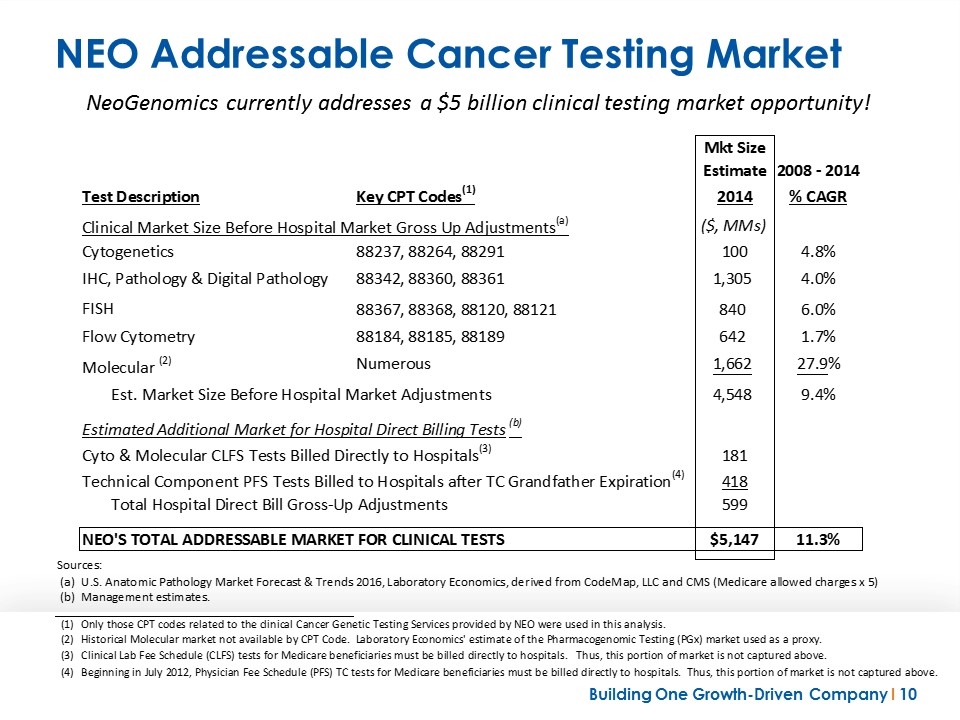
NEO Addressable Cancer Testing Market NeoGenomics currently addresses a $5 billion clinical testing market opportunity! Mkt Size Estimate 2008 - 2014 Test Description Key CPT Codes(1) 2009 2010 2011 2014 % CAGR Clinical Market Size Before Hospital Market Gross Up Adjustments(a) ($, MMs) Cytogenetics 88237, 88264, 88291 80.256405000000001 84.699870000000004 81.392859999999999 99.987530000000007 4.8% IHC, Pathology & Digital Pathology 88342, 88360, 88361 1,176.9470449999999 1,375.581835 1,587.7490749999999 1305.105892 4.2% FISH 88367, 88368, 88120, 88121 840.38067000000001 1,020.2352100000001 771.66886 839.67562499999997 6.3% Flow Cytometry 88184, 88185, 88189 703.40735999999993 787.24688999999989 859.98723999999993 641.69264499999997 1.7% Molecular (2) Numerous 505.24327 706.27372500000001 935.23361999999997 1661.588632 0.27923949876848697 Est. Market Size Before Hospital Market Adjustments 3,306.2347499999996 3,974.375300000001 4,236.316549999998 4548.0503239999998 9.4% Estimated Additional Market for Hospital Direct Billing Tests(b) Cyto & Molecular CLFS Tests Billed Directly to Hospitals(3) 66.575608000000003 87.567346500000014 109.801934 181.15699270000002 Technical Component PFS Tests Billed to Hospitals after TC Grandfather Expiration(4) 0 0 0 417.97112430000004 Total Hospital Direct Bill Gross-Up Adjustments 66.575608000000003 87.567346500000014 109.801934 599.12811700000009 NEO'S TOTAL ADDRESSABLE MARKET FOR CLINICAL TESTS 3,372.8103579999997 4,061.6048765 4,345.8335889999998 $5,147.178441 0.11288627435890342 Sources: (a) U.S. Anatomic Pathology Market Forecast & Trends 2016, Laboratory Economics, derived from CodeMap, LLC and CMS (Medicare allowed charges x 5) (b) Management estimates. (1) Only those CPT codes related to the clinical Cancer Genetic Testing Services provided by NEO were used in this analysis. (2) Historical Molecular market not available by CPT Code. Laboratory Economics' estimate of the Pharmacogenomic Testing (PGx) market used as a proxy. (3) Clinical Lab Fee Schedule (CLFS) tests for Medicare beneficiaries must be billed directly to hospitals. Thus, this portion of market is not captured above. (4) Beginning in July 2012, Physician Fee Schedule (PFS) TC tests for Medicare beneficiaries must be billed directly to hospitals. Thus, this portion of market is not captured above.
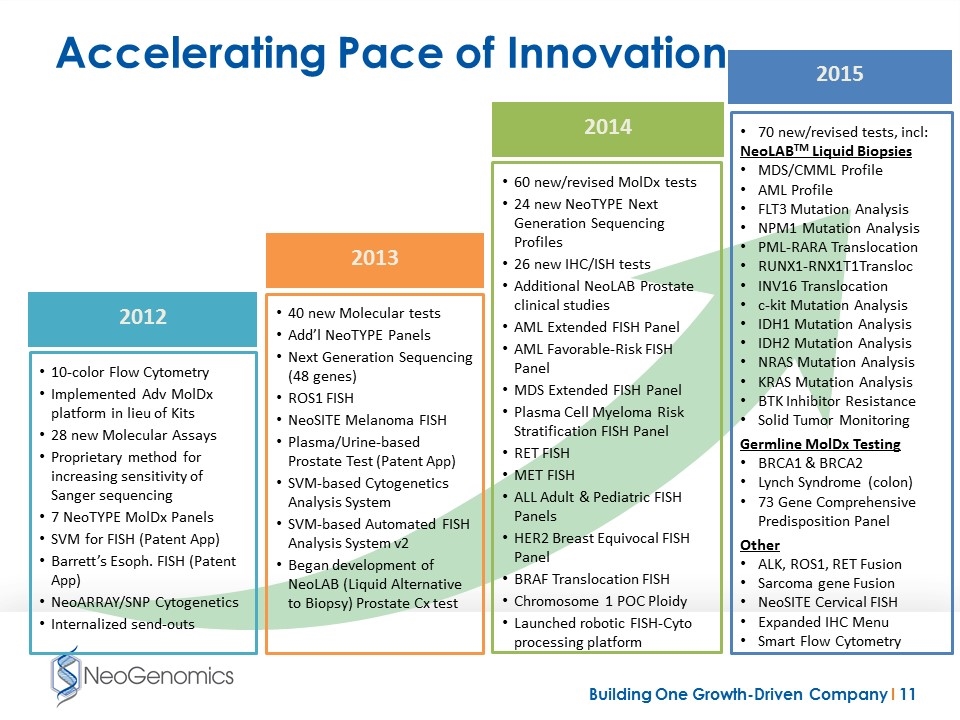
Accelerating Pace of Innovation 70 new/revised tests, incl: NeoLABTM Liquid Biopsies MDS/CMML Profile AML Profile FLT3 Mutation Analysis NPM1 Mutation Analysis PML-RARA Translocation RUNX1-RNX1T1Transloc INV16 Translocation c-kit Mutation Analysis IDH1 Mutation Analysis IDH2 Mutation Analysis NRAS Mutation Analysis KRAS Mutation Analysis BTK Inhibitor Resistance Solid Tumor Monitoring Germline MolDx Testing BRCA1 & BRCA2 Lynch Syndrome (colon) 73 Gene Comprehensive Predisposition Panel Other ALK, ROS1, RET Fusion Sarcoma gene Fusion NeoSITE Cervical FISH Expanded IHC Menu Smart Flow Cytometry 10-color Flow Cytometry Implemented Adv MolDx platform in lieu of Kits 28 new Molecular Assays Proprietary method for increasing sensitivity of Sanger sequencing 7 NeoTYPE MolDx Panels SVM for FISH (Patent App) Barrett’s Esoph. FISH (Patent App) NeoARRAY/SNP Cytogenetics Internalized send-outs 40 new Molecular tests Add’l NeoTYPE Panels Next Generation Sequencing (48 genes) ROS1 FISH NeoSITE Melanoma FISH Plasma/Urine-based Prostate Test (Patent App) SVM-based Cytogenetics Analysis System SVM-based Automated FISH Analysis System v2 Began development of NeoLAB (Liquid Alternative to Biopsy) Prostate Cx test 2015 2012 2013 60 new/revised MolDx tests 24 new NeoTYPE Next Generation Sequencing Profiles 26 new IHC/ISH tests Additional NeoLAB Prostate clinical studies AML Extended FISH Panel AML Favorable-Risk FISH Panel MDS Extended FISH Panel Plasma Cell Myeloma Risk Stratification FISH Panel RET FISH MET FISH ALL Adult & Pediatric FISH Panels HER2 Breast Equivocal FISH Panel BRAF Translocation FISH Chromosome 1 POC Ploidy Launched robotic FISH-Cyto processing platform 2014
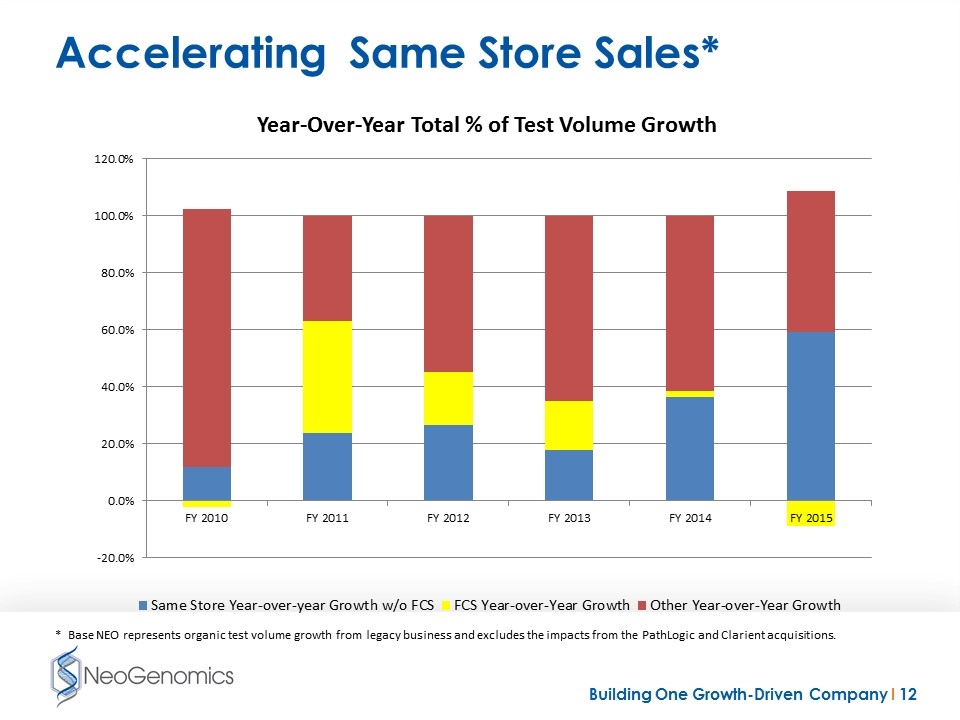
Accelerating Same Store Sales* * Base NEO represents organic test volume growth from legacy business and excludes the impacts from the PathLogic and Clarient acquisitions.
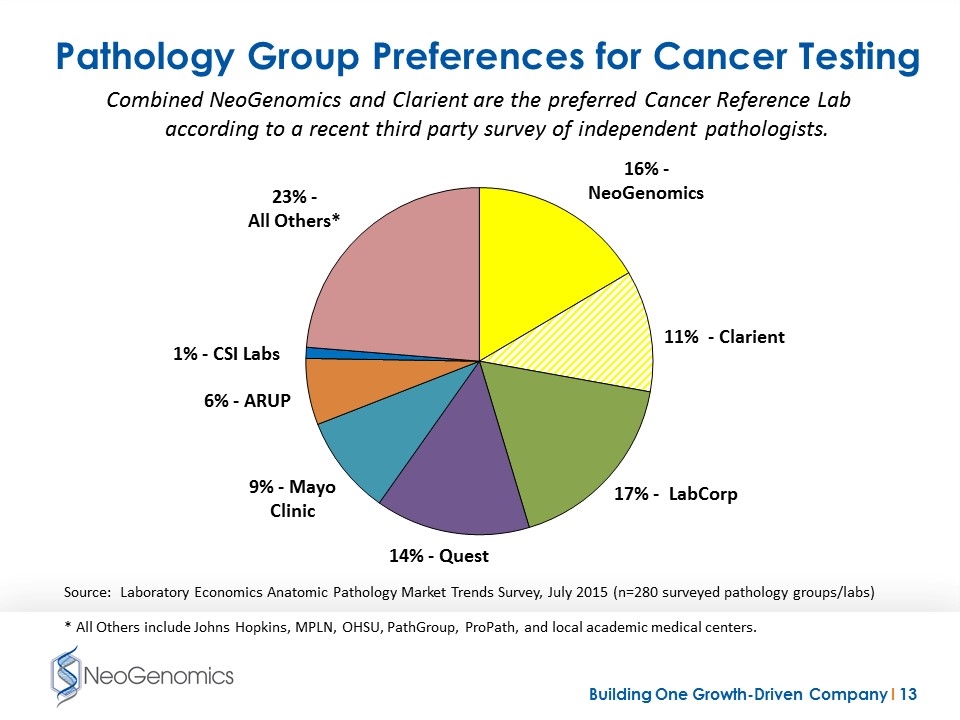
Pathology Group Preferences for Cancer Testing Source: Laboratory Economics Anatomic Pathology Market Trends Survey, July 2015 (n=280 surveyed pathology groups/labs) * All Others include Johns Hopkins, MPLN, OHSU, PathGroup, ProPath, and local academic medical centers. Combined NeoGenomics and Clarient are the preferred Cancer Reference Lab according to a recent third party survey of independent pathologists.
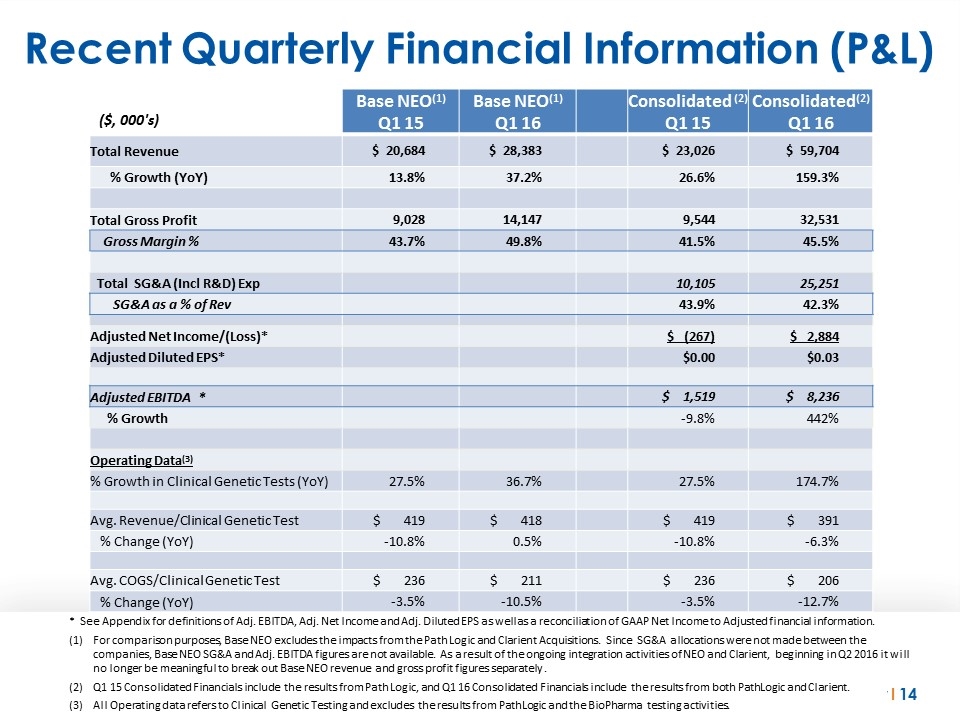
Recent Quarterly Financial Information (P&L) ($, 000's) Base NEO(1) Q1 15 Base NEO(1) Q1 16 Consolidated (2) Q1 15 Consolidated(2) Q1 16 Total Revenue $ 20,684 $ 28,383 $ 23,026 $ 59,704 % Growth (YoY) 13.8% 37.2% 26.6% 159.3% Total Gross Profit 9,028 14,147 9,544 32,531 Gross Margin % 43.7% 49.8% 41.5% 45.5% Total SG&A (Incl R&D) Exp 10,105 25,251 SG&A as a % of Rev 43.9% 42.3% Adjusted Net Income/(Loss)* $ (267) $ 2,884 Adjusted Diluted EPS* $0.00 $0.03 Adjusted EBITDA * $ 1,519 $ 8,236 % Growth -9.8% 442% Operating Data(3) % Growth in Clinical Genetic Tests (YoY) 27.5% 36.7% 27.5% 174.7% Avg. Revenue/Clinical Genetic Test $ 419 $ 418 $ 419 $ 391 % Change (YoY) -10.8% 0.5% -10.8% -6.3% Avg. COGS/Clinical Genetic Test $ 236 $ 211 $ 236 $ 206 % Change (YoY) -3.5% -10.5% -3.5% -12.7% * See Appendix for definitions of Adj. EBITDA, Adj. Net Income and Adj. Diluted EPS as well as a reconciliation of GAAP Net Income to Adjusted financial information. For comparison purposes, Base NEO excludes the impacts from the Path Logic and Clarient Acquisitions. Since SG&A allocations were not made between the companies, Base NEO SG&A and Adj. EBITDA figures are not available. As a result of the ongoing integration activities of NEO and Clarient, beginning in Q2 2016 it will no longer be meaningful to break out Base NEO revenue and gross profit figures separately . Q1 15 Consolidated Financials include the results from Path Logic, and Q1 16 Consolidated Financials include the results from both PathLogic and Clarient. All Operating data refers to Clinical Genetic Testing and excludes the results from PathLogic and the BioPharma testing activities.
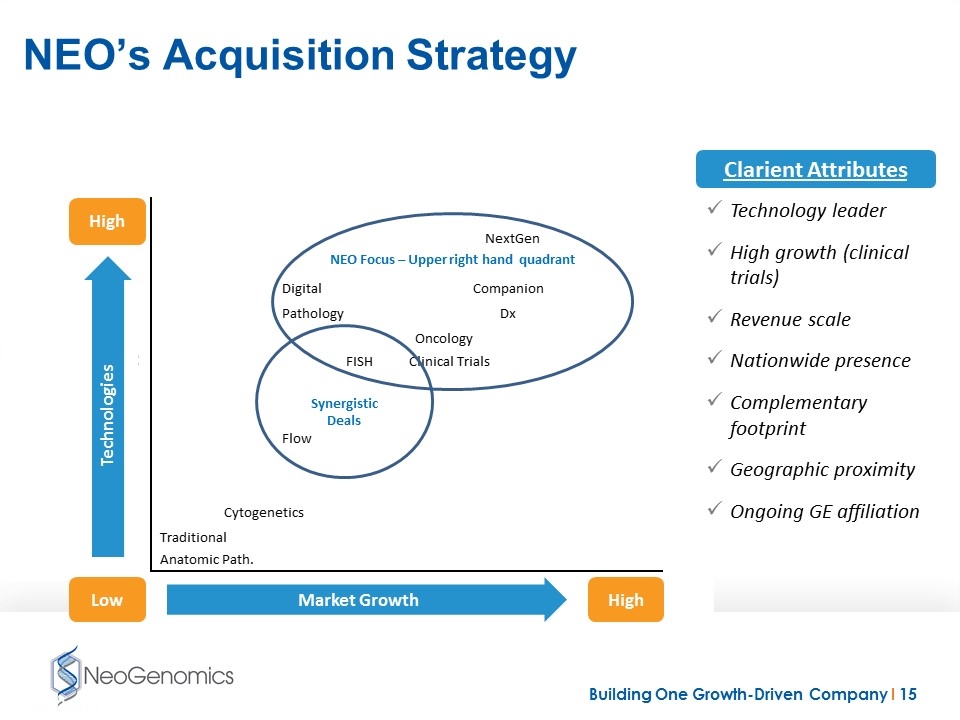
NEO’s Acquisition Strategy NEO Focus – Upper right hand quadrant Synergistic Deals High High Low Market Growth Technologies Clarient Attributes Technology leader High growth (clinical trials) Revenue scale Nationwide presence Complementary footprint Geographic proximity Ongoing GE affiliation
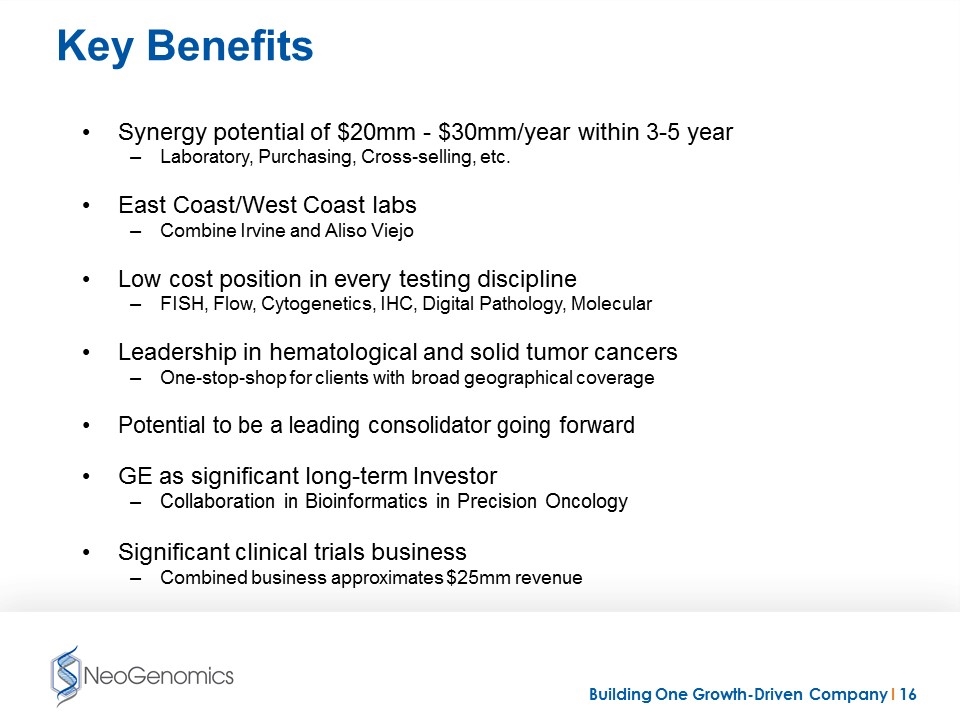
Key Benefits Synergy potential of $20mm - $30mm/year within 3-5 year Laboratory, Purchasing, Cross-selling, etc. East Coast/West Coast labs Combine Irvine and Aliso Viejo Low cost position in every testing discipline FISH, Flow, Cytogenetics, IHC, Digital Pathology, Molecular Leadership in hematological and solid tumor cancers One-stop-shop for clients with broad geographical coverage Potential to be a leading consolidator going forward GE as significant long-term Investor Collaboration in Bioinformatics in Precision Oncology Significant clinical trials business Combined business approximates $25mm revenue
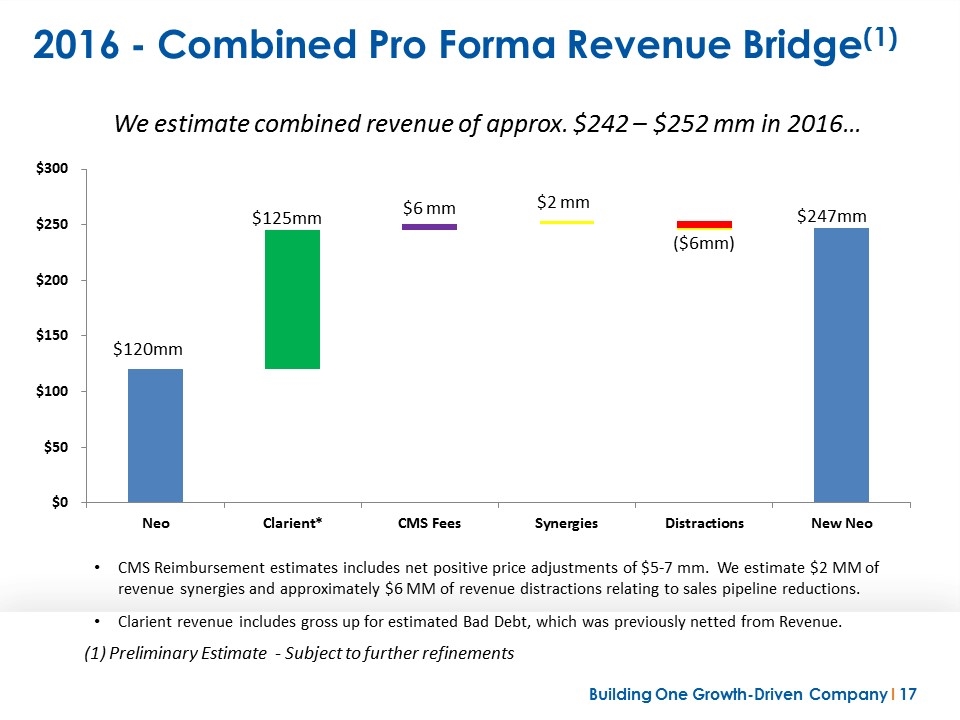
2016 - Combined Pro Forma Revenue Bridge(1) (1) Preliminary Estimate - Subject to further refinements We estimate combined revenue of approx. $242 – $252 mm in 2016… CMS Reimbursement estimates includes net positive price adjustments of $5-7 mm. We estimate $2 MM of revenue synergies and approximately $6 MM of revenue distractions relating to sales pipeline reductions. Clarient revenue includes gross up for estimated Bad Debt, which was previously netted from Revenue.
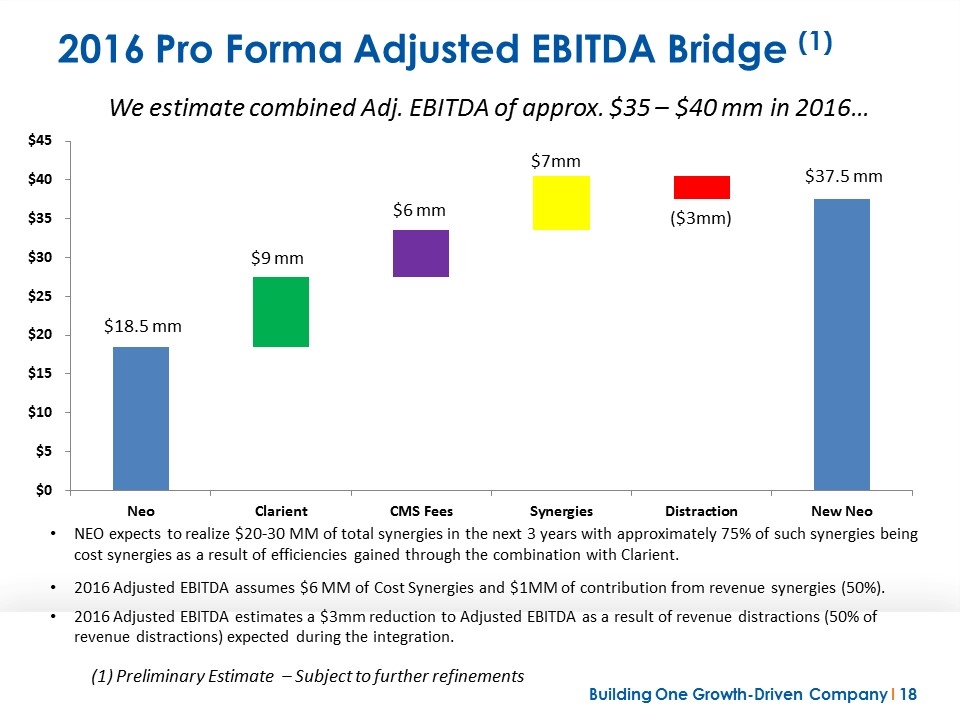
2016 Pro Forma Adjusted EBITDA Bridge (1) NEO expects to realize $20-30 MM of total synergies in the next 3 years with approximately 75% of such synergies being cost synergies as a result of efficiencies gained through the combination with Clarient. 2016 Adjusted EBITDA assumes $6 MM of Cost Synergies and $1MM of contribution from revenue synergies (50%). 2016 Adjusted EBITDA estimates a $3mm reduction to Adjusted EBITDA as a result of revenue distractions (50% of revenue distractions) expected during the integration. (1) Preliminary Estimate – Subject to further refinements We estimate combined Adj. EBITDA of approx. $35 – $40 mm in 2016…
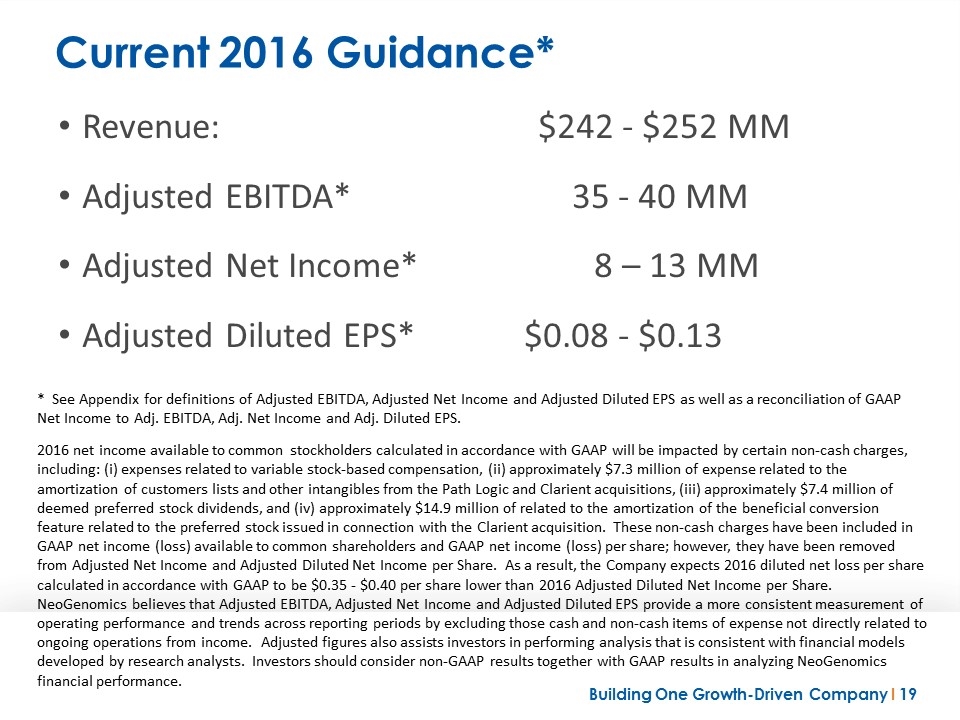
Current 2016 Guidance* Revenue:$242 - $252 MM Adjusted EBITDA* 35 - 40 MM Adjusted Net Income* 8 – 13 MM Adjusted Diluted EPS* $0.08 - $0.13 * See Appendix for definitions of Adjusted EBITDA, Adjusted Net Income and Adjusted Diluted EPS as well as a reconciliation of GAAP Net Income to Adj. EBITDA, Adj. Net Income and Adj. Diluted EPS. 2016 net income available to common stockholders calculated in accordance with GAAP will be impacted by certain non-cash charges, including: (i) expenses related to variable stock-based compensation, (ii) approximately $7.3 million of expense related to the amortization of customers lists and other intangibles from the Path Logic and Clarient acquisitions, (iii) approximately $7.4 million of deemed preferred stock dividends, and (iv) approximately $14.9 million of related to the amortization of the beneficial conversion feature related to the preferred stock issued in connection with the Clarient acquisition. These non-cash charges have been included in GAAP net income (loss) available to common shareholders and GAAP net income (loss) per share; however, they have been removed from Adjusted Net Income and Adjusted Diluted Net Income per Share. As a result, the Company expects 2016 diluted net loss per share calculated in accordance with GAAP to be $0.35 - $0.40 per share lower than 2016 Adjusted Diluted Net Income per Share. NeoGenomics believes that Adjusted EBITDA, Adjusted Net Income and Adjusted Diluted EPS provide a more consistent measurement of operating performance and trends across reporting periods by excluding those cash and non-cash items of expense not directly related to ongoing operations from income. Adjusted figures also assists investors in performing analysis that is consistent with financial models developed by research analysts. Investors should consider non-GAAP results together with GAAP results in analyzing NeoGenomics financial performance.
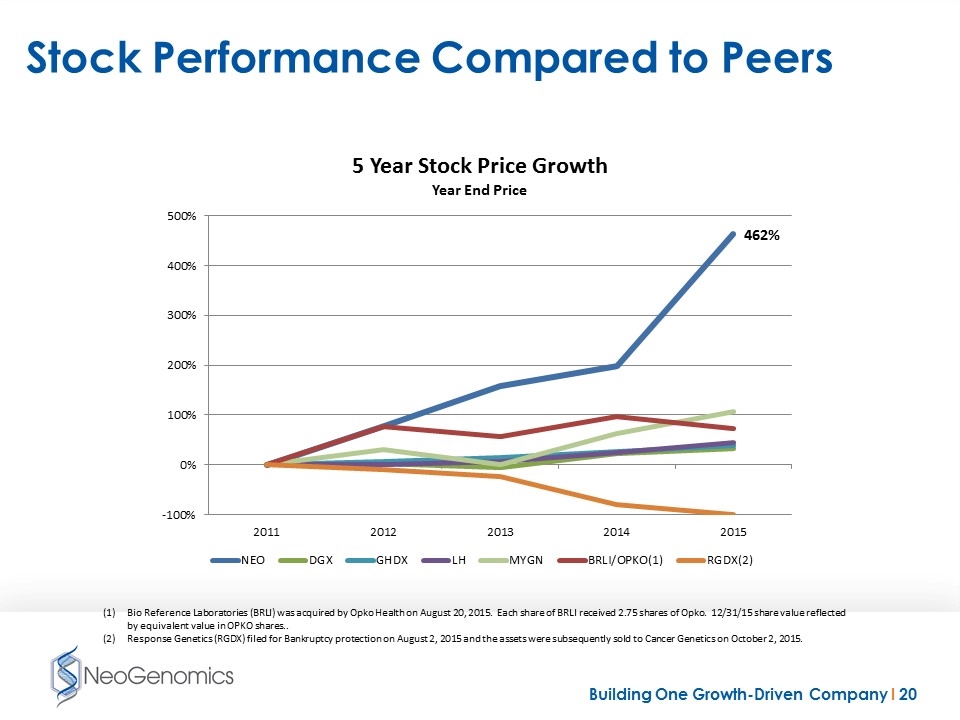
Stock Performance Compared to Peers Bio Reference Laboratories (BRLI) was acquired by Opko Health on August 20, 2015. Each share of BRLI received 2.75 shares of Opko. 12/31/15 share value reflected by equivalent value in OPKO shares.. Response Genetics (RGDX) filed for Bankruptcy protection on August 2, 2015 and the assets were subsequently sold to Cancer Genetics on October 2, 2015.

NeoGenomics Vision and Goals Leadership Goals: Be the leading oncology-focused testing and information company in the World. Innovate aggressively to advance precision medicine. Be the highest quality and lowest cost provider in each product area Competencies: Outstanding Scientific, Medical and Informatics expertise. Partnerships for efficiency/effectiveness across health-care care continuum Disciplined process management and quality systems. Entrepreneurial, values-driven culture. Financial Performance Goals: Consistent and sustainable double-digit revenue growth. Clinical Trials > 15% of revenue. Adjusted EBITDA margin of 20-25% when annual revenue >$300 MM.

Appendix
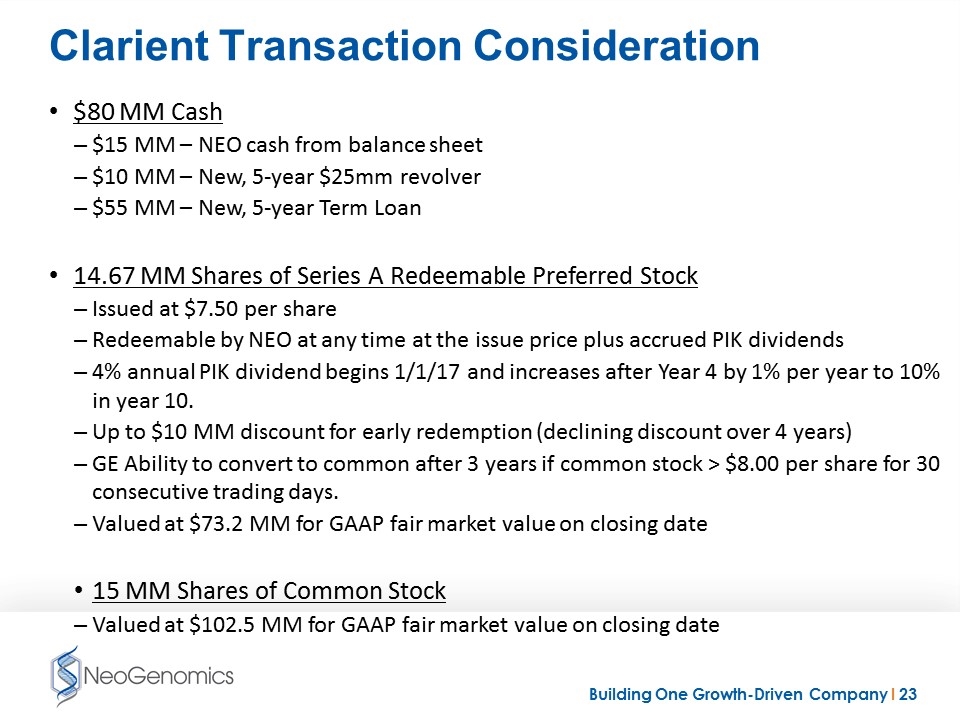
Clarient Transaction Consideration $80 MM Cash $15 MM – NEO cash from balance sheet $10 MM – New, 5-year $25mm revolver $55 MM – New, 5-year Term Loan 14.67 MM Shares of Series A Redeemable Preferred Stock Issued at $7.50 per share Redeemable by NEO at any time at the issue price plus accrued PIK dividends 4% annual PIK dividend begins 1/1/17 and increases after Year 4 by 1% per year to 10% in year 10. Up to $10 MM discount for early redemption (declining discount over 4 years) GE Ability to convert to common after 3 years if common stock > $8.00 per share for 30 consecutive trading days. Valued at $73.2 MM for GAAP fair market value on closing date 15 MM Shares of Common Stock Valued at $102.5 MM for GAAP fair market value on closing date
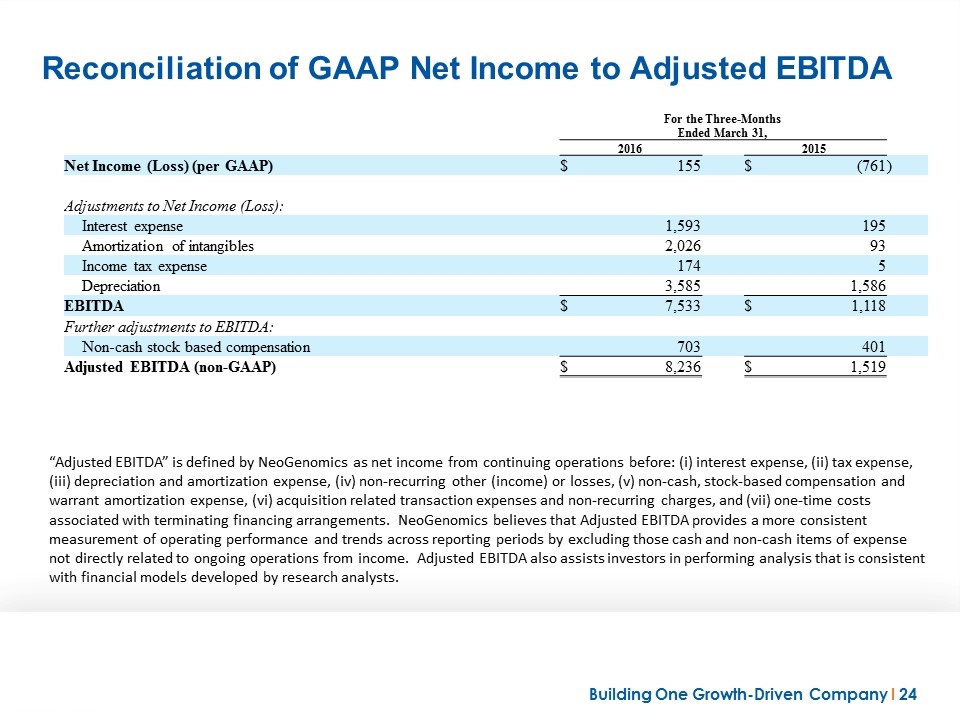
Reconciliation of GAAP Net Income to Adjusted EBITDA “Adjusted EBITDA” is defined by NeoGenomics as net income from continuing operations before: (i) interest expense, (ii) tax expense, (iii) depreciation and amortization expense, (iv) non-recurring other (income) or losses, (v) non-cash, stock-based compensation and warrant amortization expense, (vi) acquisition related transaction expenses and non-recurring charges, and (vii) one-time costs associated with terminating financing arrangements. NeoGenomics believes that Adjusted EBITDA provides a more consistent measurement of operating performance and trends across reporting periods by excluding those cash and non-cash items of expense not directly related to ongoing operations from income. Adjusted EBITDA also assists investors in performing analysis that is consistent with financial models developed by research analysts. For the Three-Months Ended March 31, 2016 2015 Net Income (Loss) (per GAAP) $ 155 $ (761 ) Adjustments to Net Income (Loss): Interest expense 1,593 195 Amortization of intangibles 2,026 93 Income tax expense 174 5 Depreciation 3,585 1,586 EBITDA $ 7,533 $ 1,118 Further adjustments to EBITDA: Non-cash stock based compensation 703 401 Adjusted EBITDA (non-GAAP) $ 8,236 $ 1,519
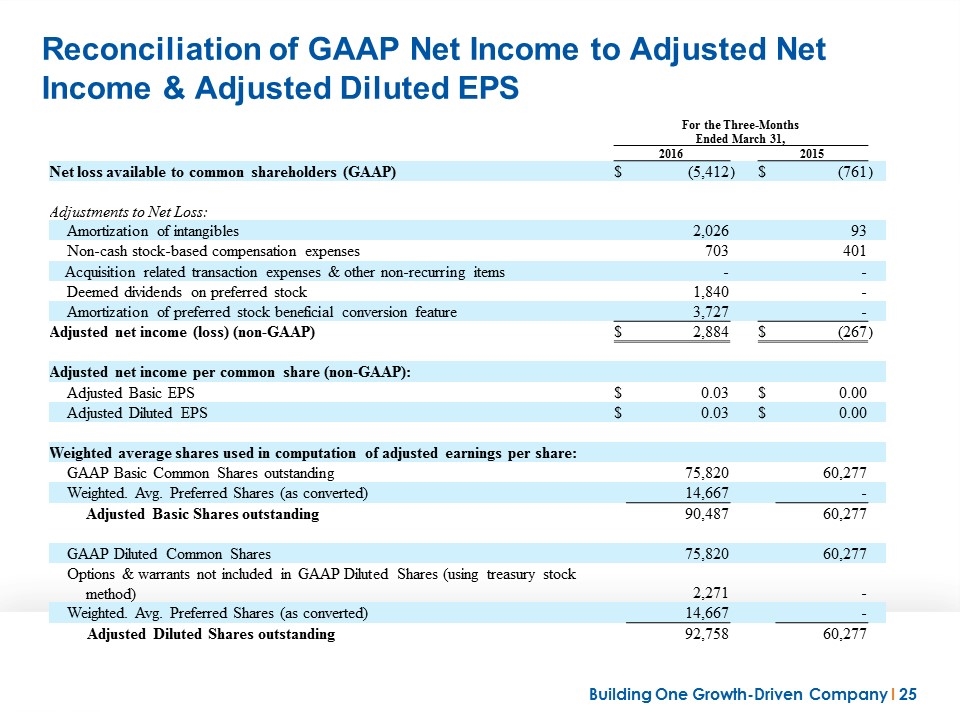
Reconciliation of GAAP Net Income to Adjusted Net Income & Adjusted Diluted EPS For the Three-Months Ended March 31, 2016 2015 Net loss available to common shareholders (GAAP) $ (5,412 ) $ (761 ) Adjustments to Net Loss: Amortization of intangibles 2,026 93 Non-cash stock-based compensation expenses 703 401 Acquisition related transaction expenses & other non-recurring items - - Deemed dividends on preferred stock 1,840 - Amortization of preferred stock beneficial conversion feature 3,727 - Adjusted net income (loss) (non-GAAP) $ 2,884 $ (267 ) Adjusted net income per common share (non-GAAP): Adjusted Basic EPS $ 0.03 $ 0.00 Adjusted Diluted EPS $ 0.03 $ 0.00 Weighted average shares used in computation of adjusted earnings per share: GAAP Basic Common Shares outstanding 75,820 60,277 Weighted. Avg. Preferred Shares (as converted) 14,667 - Adjusted Basic Shares outstanding 90,487 60,277 GAAP Diluted Common Shares 75,820 60,277 Options & warrants not included in GAAP Diluted Shares (using treasury stock method) 2,271 - Weighted. Avg. Preferred Shares (as converted) 14,667 - Adjusted Diluted Shares outstanding 92,758 60,277
“Adjusted Net Income” is defined by NeoGenomics as net income available to common shareholders from continuing operations plus: (i) amortization of customer lists and other intangible assets, (ii) non-cash, stock-based compensation and warrant amortization expense, (iii) non-recurring other (income) or losses, (iv) acquisition related transaction expenses and non-recurring charges, (v) deemed dividends on preferred stock, and (vi) amortization of preferred stock beneficial conversion feature. NeoGenomics believes that Adjusted Net Income provides a more consistent measurement of operating performance and trends across reporting periods by excluding those cash and non-cash items of expense not directly related to ongoing operations from net income. Adjusted Net Income also assists investors in performing analysis that is consistent with financial models developed by research analysts. “Adjusted EPS” is calculated using Adjusted Basic Shares and Adjusted Diluted Shares outstanding. Adjusted Basic Shares and Adjusted Diluted Shares include the weighted average number of common shares that would be outstanding if the preferred stock were converted into common stock on the original issue date based on the number of days such common shares would have been outstanding in the reporting period. In addition, If GAAP Net Income is negative and Adjusted Net Income is positive, Adjusted Diluted Shares will also include any options or warrants that would be outstanding as dilutive instruments using the treasury stock method. Adjusted EBITDA, Adjusted Net Income and Adjusted EPS as defined by NeoGenomics are not measurements under GAAP and may differ from non-GAAP measures used by other companies. There are limitations inherent in non-GAAP financial measures such as Adjusted EBITDA, Adjusted Net Income and Adjusted EPS because they exclude a variety of charges and credits that are required to be included in a GAAP presentation, and do not therefore present the full measure of NeoGenomics recorded costs against its net revenue. Accordingly, investors should consider non-GAAP results together with GAAP results in analyzing NeoGenomics financial performance. Reconciliation of GAAP Net Income to Adjusted Figures