10-K: Annual report pursuant to Section 13 and 15(d)
Published on March 13, 2018
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2017
or
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-35756
NEOGENOMICS, INC.
(Exact name of registrant as specified in its charter)
|
Nevada |
|
74-2897368 |
|
(State or other jurisdiction of incorporation or organization) |
|
(IRS Employer Identification No.) |
12701 Commonwealth Drive, Suite 9, Fort Myers, FL 33913
(Address of principal executive offices, Zip code)
(239) 768-0600
(Registrant’s telephone number, including area code)
|
Securities registered pursuant to Section 12(b) of the Act: |
|
Name of each exchange on which registered: |
|
Common Stock, par value $0.001 per share |
|
NASDAQ Capital Market |
Securities registered pursuant to Section 12(g) of the Act: Common Stock par value $0.001 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
|
Accelerated filer |
☑ |
|
Non-accelerated filer |
☐ |
(Do not check if a smaller reporting company) |
Smaller Reporting Company |
☐ |
|
|
|
|
Emerging Growth Company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act): ☐ Yes ☒ No
As of June 30, 2017, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was approximately $653.1 million, based on the closing price of the registrant’s common stock of $8.96 per share on June 30, 2017.
The number of shares outstanding of the registrant’s Common Stock, par value $0.001 per share, as of March 5, 2018: 80,507,094
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Proxy Statement for its 2018 Annual Meeting of stockholders are incorporated by reference into Part III of this Annual Report on Form 10-K.
NEOGENOMICS, INC.
For the Fiscal Year Ended December 31, 2017
Table of Contents
|
|
|
|
Page |
|
|
PART I |
|
|
|
|
|
Item 1. |
|
|
4 |
|
|
Item 1A. |
|
|
24 |
|
|
Item 1B. |
|
|
44 |
|
|
Item 2. |
|
|
45 |
|
|
Item 3. |
|
|
45 |
|
|
Item 4. |
|
|
45 |
|
|
|
|
|
|
|
|
PART II |
|
|
|
|
|
Item 5. |
|
|
46 |
|
|
Item 6. |
|
|
49 |
|
|
Item 7. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
51 |
|
Item 7A. |
|
|
78 |
|
|
Item 8. |
|
|
79 |
|
|
Item 9. |
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
|
125 |
|
Item 9A. |
|
|
125 |
|
|
Item 9B. |
|
|
125 |
|
|
|
|
|
|
|
|
PART III |
|
|
|
|
|
Item 10. |
|
|
126 |
|
|
Item 11. |
|
|
126 |
|
|
Item 12. |
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
126 |
|
Item 13. |
|
Certain Relationships and Related Transactions, and Director Independence |
|
126 |
|
Item 14. |
|
|
126 |
|
|
|
|
|
|
|
|
PART IV |
|
|
|
|
|
Item 15. |
|
|
127 |
|
|
|
|
|
|
|
|
|
131 |
|||
NeoGenomics, NeoLAB and NeoTYPE are our registered trademarks, and FlexREPORT is our trademark. Any other trademarks, registered marks and trade names appearing in this annual report on Form 10-K are the property of their respective holders.
2
NEOGENOMICS, INC.
The information in this Annual Report on Form 10-K contains “forward-looking statements” and information within the meaning of Section 27A of the Securities Act of 1933, as amended, or the “Securities Act”, and Section 21E of the Securities Exchange Act of 1934, as amended, or the “Exchange Act”, which are subject to the “safe harbor” created by those sections. These forward-looking statements include, but are not limited to, statements concerning our strategy, future operations, future financial position, future revenues, changing reimbursement levels from government payers and private insurers, projected costs, prospects and plans and objectives of management. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. These forward-looking statements involve known and unknown risks and uncertainties that could cause our actual results, performance or achievements to differ materially from those expressed or implied by the forward-looking statements, including, without limitation, the risks set forth in Part I, Item 1A, “Risk Factors” in this Annual Report on Form 10-K and in our other filings with the Securities and Exchange Commission, or “SEC”.
Forward-looking statements include, but are not limited to, statements about:
|
• |
Our ability to implement our business strategy; |
|
• |
The expected reimbursement levels from governmental payers and private insurers and proposed changes to those levels; |
|
• |
The application, to our business and the services we provide, of existing laws, rules and regulations, including without limitation, Medicare laws, anti-kickback laws, Health Insurance Portability and Accountability Act of 1996 regulations, state medical privacy laws, federal and state false claims laws and corporate practice of medicine laws; |
|
• |
Regulatory developments in the United States including downward pressure on health care reimbursement; |
|
• |
Our ability to maintain our license under the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”); |
|
• |
Food and Drug Administration, or FDA regulation of Laboratory Developed Tests (“LDTs”); |
|
• |
Failure to timely or accurately bill for our services; |
|
• |
Our ability to expand our operations and increase our market share; |
|
• |
Our ability to expand our service offerings by adding new testing capabilities; |
|
• |
Our ability to meet our future capital requirements; |
|
• |
Our ability to manage our indebtedness; |
|
• |
Our ability to protect our intellectual property from infringement; |
|
• |
Our ability to integrate future acquisitions and costs related to such acquisitions; |
|
• |
The impact of internalization of testing by customers; |
|
• |
Our ability to maintain service levels and compete with other diagnostic laboratories; |
|
• |
Our ability to hire and retain sufficient managerial, sales, clinical and other personnel to meet our needs; |
|
• |
Our ability to successfully scale our business, including expanding our facilities, our backup systems and infrastructure; and |
|
• |
The accuracy of our estimates regarding reimbursement, expenses, future revenues and capital requirements. |
These forward-looking statements represent our management’s beliefs and assumptions only as of the date of this Annual Report. You should read this Annual Report and the documents that we reference in this Annual Report and have filed as exhibits, completely and with the understanding that our actual future results may be materially different from what we expect.
Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
3
NEOGENOMICS, INC
ITEM 1. BUSINESS
NeoGenomics, Inc., a Nevada corporation (referred to individually as the “Parent Company” or collectively with its subsidiaries as “NeoGenomics”, “we”, “us”, “our” or the “Company” in this Annual Report) is the registrant for SEC reporting purposes. Our common stock is listed on the NASDAQ Capital Market under the symbol “NEO”.
Overview
We operate a network of cancer-focused genetic testing laboratories in the United States as well as a laboratory in Switzerland. Our mission is to improve patient care through exceptional genetic and molecular testing services. Our vision is to become the World’s leading cancer testing and information company by delivering uncompromising quality, exceptional service and innovative solutions.
As of December 31, 2017, the Company has laboratory locations in Ft. Myers and Tampa, Florida; Aliso Viejo and Fresno California; Houston, Texas; Nashville, Tennessee; and Rolle, Switzerland, and currently offers the following types of genetic and molecular testing services:
|
|
a) |
Cytogenetics - the study of normal and abnormal chromosomes and their relationship to disease. It involves looking at the chromosome structure to identify changes from patterns seen in normal chromosomes. Cytogenetic studies are often utilized to answer diagnostic, prognostic and predictive questions in the treatment of hematological malignancies. |
|
|
b) |
Fluorescence In-Situ Hybridization (“FISH”) - a branch of cancer genetics that focuses on detecting and locating the presence or absence of specific DNA sequences and genes on chromosomes. FISH helps bridge abnormality detection between the chromosomal and DNA sequence levels. The technique uses fluorescent probes that bind to only those parts of the chromosome with which they show a high degree of sequence similarity. Fluorescence microscopy is used to visualize the fluorescent probes bound to the chromosomes. FISH can be used to help identify a number of gene alternations, such as amplification, deletions, and translocations. |
|
|
c) |
Flow cytometry - a rapid way to measure the characteristics of cell populations. Cells from peripheral blood, bone marrow aspirate, lymph nodes, and other areas are labeled with selective fluorescent antibodies and analyzed as they flow in a fluid stream through a beam of light. The properties measured in these antibodies include the relative size, relative granularity or internal complexity, and relative fluorescence intensity. These fluorescent antibodies bind to specific cell surface antigens and are used to identify malignant cell populations. Flow cytometry is typically performed in diagnosing a wide variety of leukemia and lymphoma neoplasms. Flow cytometry is also used to monitor patients through therapy to determine whether the disease burden is increasing or decreasing, otherwise known as minimal residual disease monitoring. |
|
|
d) |
Immunohistochemistry (“IHC”) and Digital Imaging – Refers to the process of localizing proteins in cells of a tissue section and relies on the principle of antibodies binding specifically to antigens in biological tissues. IHC is widely used in the diagnosis of abnormal cells such as those found in cancerous tumors. Specific surface cytoplasmic or nuclear markers are characteristic of cellular events such as proliferation or cell death (apoptosis). IHC is also widely used to understand the distribution and localization of differentially expressed proteins. Digital imaging allows clients to see and utilize scanned slides and perform quantitative analysis for certain stains. Scanned slides are received online in real time and can be previewed often a full day before the glass slides can be shipped back to clients. |
|
|
e) |
Molecular testing - a rapidly growing cancer diagnostic tool focusing on the analysis of DNA and RNA, as well as the structure and function of genes at the molecular level. Molecular testing employs multiple technologies including DNA fragment length analysis, real-time polymerase chain reaction (“RT-PCR”) RNA analysis, bi-directional Sanger sequencing analysis, and Next-Generation Sequencing (“NGS”). |
|
|
f) |
Pathology consultation - services provided for clients in which our pathologists review surgical samples on a consultative basis. NeoGenomics expert pathologists often assist our client pathologists on their most difficult and complex cases. |
4
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
Operating Segments
We analyzed our reporting structure in 2017, including the information available to our Chief Operating Decision Maker and the information used to make strategic decisions. Prior to 2017, our operations were reported as one consolidated segment. Based on our 2017 analysis and due to changes made in the fourth quarter of 2017, we began reporting our operations in two segments; Clinical Services and Pharma Services.
In 2017, our Clinical Services segment accounted for 90% of consolidated revenues and our Pharma Services segment accounted for 10% of our consolidated revenues. For further financial information about these segments, see Note Q to our Consolidated Financial Statements included in this Annual Report.
Clinical Services Segment
The clinical cancer testing services we offer to community-based pathologists are designed to be a natural extension of, and complementary to, the services that they perform within their own practices. We believe our relationship as a non-competitive partner to community-based pathology practices, hospital pathology labs and academic centers empowers them to expand their breadth of testing and provide a menu of services that matches or exceeds the level of service found in any center of excellence around the world. Community-based pathology practices and hospital pathology labs may order certain testing services on a technical component only (“TC” or “tech-only”) basis, which allows them to participate in the diagnostic process by performing the professional component (“PC”) interpretation services without having to hire laboratory technologists or purchase the sophisticated equipment needed to perform the technical component of the tests. We also support our pathology clients with interpretation and consultative services using our own specialized team of pathologists for difficult or complex cases and provide overflow interpretation services when requested by clients.
In addition, we may directly serve oncology, dermatology, urology and other clinician practices that prefer to have a direct relationship with a laboratory for cancer-related genetic and molecular testing services. We typically service these types of clients with a comprehensive service offering where we perform both the technical and professional components of the tests ordered. In certain instances larger clinician practices have begun to internalize pathology interpretation services, and our “tech-only” service offering allows these larger clinician practices to also participate in the diagnostic process by performing the PC interpretation services on TC testing performed by NeoGenomics. In these instances NeoGenomics will typically provide all of the more complex, Molecular testing services.
Pharma Services Segment
Our Pharma Services segment supports pharmaceutical firms in their drug development programs by supporting various clinical trials and research. This portion of our business often involves working with the pharmaceutical firms (sponsors) on study design as well as performing the required testing. Our medical team often advises the sponsor and works closely with them as specimens are received from the enrolled sites. We also work on developing tests that will be used as part of a companion diagnostic to determine patients’ response to a particular drug. As studies unfold, our clinical trials team reports the data and often provide key analysis and insights back to the sponsors.
Our Pharma Services segment provides comprehensive testing services in support of our pharmaceutical clients’ oncology programs from discovery to commercialization. In biomarker discovery, our aim is to help our customers discover the right content. We help our customers develop a biomarker hypothesis by recommending an optimal platform for molecular screening and backing our discovery tools with the informatics to capture meaningful data. In other pre and non-clinical work, we can use our platforms to characterize markers of interest. Moving from discovery to development, we help our customers refine their biomarker strategy and, if applicable, develop a companion diagnostic pathway using the optimal technology for large-scale clinical trial testing.
Whether serving as the single contract research organization or partnering with one, our Pharma Services team provides significant technical expertise, working closely with our customers to support each stage of clinical trial
5
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
development. Each trial we support comes with rapid turnaround time, dedicated project management and quality assurance oversight. We have experience in supporting submissions to the Federal Drug Administration (“FDA”) for companion diagnostics. Our Pharma Services strategy is focused on helping bring more effective oncology treatments to market through providing world class laboratory services in oncology to key pharmaceutical companies in the industry.
Our Pharma Services revenue consists of three revenue streams:
|
|
• |
Clinical trials and research; |
|
|
• |
Validation laboratory services; and |
|
|
• |
Data services |
Markets
The medical testing laboratory market can be broken down into three primary markets:
|
|
• |
Clinical Pathology testing; |
|
|
• |
Anatomic Pathology testing; and |
|
|
• |
Genetic and Molecular testing |
Clinical Pathology testing covers high volume, highly automated, lower complexity tests on easily procured specimens such as blood and urine. Clinical lab tests often involve testing of a less urgent nature, for example, cholesterol testing and testing associated with routine physical exams.
Anatomic Pathology testing involves evaluation of tissue, as in surgical pathology, or cells as in cytopathology. The most widely performed Anatomic Pathology procedures include the preparation and interpretation of pap smears, skin biopsies, and tissue biopsies.
Genetic and molecular testing typically involves analyzing chromosomes, genes, proteins and/or DNA/RNA sequences for abnormalities. Genetic and molecular testing requires highly specialized equipment and credentialed individuals (typically M.D. or Ph.D. level) to certify results and typically yields the highest reimbursement levels of the three market segments.
NeoGenomics operates primarily in the Genetic and Molecular testing market. We also act as a reference laboratory supplying anatomic pathology testing. NeoGenomics typically does not compete in the Clinical Pathology testing market.
The field of cancer genetics is evolving rapidly and new tests are being developed at an accelerated pace. Based on medical and scientific discoveries over the last decade, cancer testing falls into one of three categories: diagnostic testing, prognostic testing and predictive testing. Of the three, the fastest growing area is predictive testing, which is utilized by clinicians to predict a patient’s response to the various treatment options in order to deliver “personalized or precision medicine” that is optimized to that patient’s particular circumstances. Personalized or precision medicine allows clinicians to know if a patient will or will not respond to certain medications like Herceptin, Keytruda and Opdivo. This saves the healthcare system money by ensuring that expensive cancer drugs are only given to those who will benefit from them. This type of testing improves patient care and potentially saves lives by identifying optimized therapies much more rapidly than what was possible in previous years.
The United States market for genetic and molecular testing is divided among numerous laboratories. Many of these laboratories are attached to academic institutions and primarily provide clinical services to their affiliated university hospitals and associated physicians.
We believe several key factors are influencing the rapid growth in the market for cancer testing: (i) every year, more and more genes and genomic pathways are implicated in the development and/or clinical course of cancer; (ii) cancer is primarily a disease of the elderly - one in four senior citizens is likely to develop some form of cancer during the rest of their lifetime once they turn sixty, and now that the baby boomer generation has started to reach
6
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
this age range, the incidence rates of cancer are rising; (iii) increasingly, new drugs are being targeted to certain cancer subtypes and pathways which require companion diagnostic testing; (iv) patient and payer awareness of the value of genetic and molecular testing; (v) decreases in the cost of performing genetic and molecular testing; (vi) increased coverage from third party payers and Medicare for such testing; and (vii) the health insurance coverage to uninsured Americans under the Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act, each enacted in March 2010. These factors have driven significant growth in the market for this type of testing. We estimate a $12-14 billion total market opportunity for cancer testing in the United States, and we estimate that about $5-7 billion of this market is made up of genetic and molecular testing with the remaining portion derived from more traditional anatomic pathology testing services that are complementary to and often ordered with the genetic and molecular testing services we offer.
2018 Focus Areas: Strengthen Our World-Class Culture, Provide Uncompromising Quality and Pursue Exceptional Service and Growth
We are committed to being an innovative leader in our industry. Over the past year, we have grown our business domestically and have expanded our presence internationally. Our plans for 2018 include many initiatives to continue our strong organic growth by gaining market share and introducing new tests. In addition, we expect to realize growth from the expansion of our Pharma business in Europe as well as in the United States. We expect these initiatives to continue to position our Company to be the World’s leading cancer testing and information company.
Strengthen Our World-Class Culture
Our belief is that a culture of motivated and engaged employees will deliver superior service to our clients. We are focused on continuing to strengthen our culture by improving teamwork, which will enable our Company to work more coherently and efficiently. We will also emphasize effective communication techniques through cross functional initiatives. We introduced initiatives and implemented targeted dialogue between management and employees in 2017 and will continue this going forward. Part of this initiative included selecting employees who were given the opportunity to talk to our CEO in a small group setting designed to foster two-way communication.
Communication is a key element in our high performance culture. Through effective communication we facilitate our employees’ understanding of our Company’s priorities and how they contribute to the Company’s overall objectives. We believe our employee retention rate is above average for the laboratory industry and continuing to strengthen our culture will enable us to continually recruit and retain talented employees.
Enhancing our culture to closely align with the values of our Company is a key priority. We plan to implement Talent Success Profiles to develop leaders and ensure that we are creating opportunities for the development and mobility of our employees. We will focus on mentoring and training opportunities to enhance and capitalize on the talent within our Company. We believe these initiatives will foster a culture of accountability and empowerment. We also believe these initiatives are necessary to ensure the success of our Company.
Provide Uncompromising Quality
Maintaining the highest quality laboratory operations and service levels has enabled us to consistently grow our business. We have been successful in retaining clients while also gaining market share. Our initiatives for 2018 will promote continuous process improvement to ensure that we maintain our high level of quality within our organization.
We plan to continue to grow a culture of quality through company-wide leadership, training and employee engagement initiatives. Through training, we aim to empower our employees to understand the importance of quality and how to ensure quality in their respective function. We will challenge employees to identify quality issues and find solutions and will recognize individuals and teams for providing quality service. Through employee engagement, we will motivate our employees to exceed our client’s expectations.
7
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
Our laboratory teams will focus on quality by improving corrective and preventative metrics in the laboratory. This is expected to result in increased product and process understanding, improvements in processes and increased efficiency. We also believe these improvements will enable us to continue reducing our cost per test.
In 2016, we began work on our next generation Laboratory Information System, or LIS, and have implemented this system in our Pharma Services business in 2017. We will continue to develop this system in 2018 and believe it will increase efficiency and productivity. It also improves the quality of our services by enabling our Pharma services clients the ability to track each step through the laboratory process.
Pursue Exceptional Service and Growth
We will continue to pursue market share gains by providing high complexity, cancer-related laboratory testing services to hospitals, community-based pathology practices, academic centers, clinicians, and Pharmaceutical companies in the United States and Europe. We will strive to improve our services and achieve long term profitable growth by developing cross functional teams to analyze the unique requirements of key market segments. We will engage our customers within these segments and analyze our strengths, weaknesses and threats to find ways to further drive growth and pursue excellent service. We will continue to seek customer feedback through our rigorous survey process, assess our customer’s satisfaction with our services, and develop plans for improvement.
While our client retention rate is excellent, in 2018 we will focus on continuing to provide consistently high service levels while engaging our customers. In addition, we will work to maintain our broad and innovative test menu of molecular, immunohistochemistry, and other testing, which has helped make us a “one stop shop” for many clients who value that all of their testing can be sent to one laboratory.
Our plans for 2018 include reimbursement and legislative strategies to drive profitable growth. We are closely monitoring changes in legislation, are taking specific actions and establishing detailed plans to be prepared for possible changes in legislation.
We expect that our expansion into Europe will fuel profitable growth in our Pharma Services business in the long-term. In addition, we are currently expanding our laboratory facility in Houston, Texas due to increased demand for Pharma Services.
We will continue to look for growth opportunities through mergers and/or acquisitions and are focused on strategic opportunities that would be complementary to our menu of services and would increase our earnings and cash flow in the short to medium timeframe.
Competitive Strengths
Turnaround Times
In our Clinical Services segment, we strive to provide industry leading turnaround times for test results to our clients nationwide. By providing information to our clients in a rapid manner, physicians can begin treating their patients as soon as possible. We believe our historical average 4-5 day turnaround time for our cytogenetics testing services, 3-4 day turnaround time for FISH testing services, 7 day turnaround time for molecular testing, and 1 day turnaround time for flow cytometry and pathology testing services are industry leading benchmarks for national laboratories.
Our consistent timeliness of results is a competitive strength and a driver of additional testing requests by our referring physicians. Rapid turnaround times allow for the performance of other adjunctive tests within an acceptable diagnosis window in order to augment or confirm results and more fully inform treatment options. We believe that fast turnaround times are a key differentiator versus other national laboratories, and our clients often cite them as a key factor in their relationship with us.
Fast response time is also critical to customer satisfaction in our Pharma Services segment. We work with the sponsors to set up the studies quickly and to provide rapid turnaround on the testing results once the samples from the study enrollees arrive at the laboratory. Final transmissions of data are also critical to sponsors who are often working on their own submissions to the FDA for approval of drug compounds. We believe that our rapid turnaround time on testing and our project milestones are a key differentiator in the Pharma Services segment.
8
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
World-class Medical and Scientific Team
Our team of medical professionals and Ph.Ds. are specialists in the field of genetics, oncology and pathology. As of December 31, 2017, we employed, or contracted with approximately 30 full-time M.D.s and Ph.Ds. We have many nationally world renowned pathologists on staff, which is a key differentiator from many smaller laboratories. Our clinical customers look to our staff and their expertise and they often call our medical team on challenging cases. For our Pharma Services segment, many sponsors work with our medical team on their study design and on the interpretation of results from the studies. Again, our medical team is a key differentiator as we have a depth of medical expertise that many other laboratories cannot offer to Pharmaceutical companies.
Extensive Tech-Only Service Offerings
We believe that NeoGenomics currently has the most extensive menu of tech-only FISH services in the country as well as extensive and advanced tech-only flow cytometry and IHC testing services. These types of testing services allow the professional interpretation component of a test to be performed and billed separately by our physician clients. Our FISH, flow cytometry and other tech-only service offerings allow properly trained and credentialed community-based pathologists to extend their own practices by performing professional interpretations services, which allows them to better service the needs of their local clientele without the need to invest in the lab equipment and personnel required to perform the technical component of genetic and molecular testing.
Our tech-only services are designed to give pathologists the option to choose, on a case by case basis, whether they want to order just the technical information and images relating to a specific test so they can perform the professional interpretation, or order global services and receive a comprehensive test report which includes a NeoGenomics pathologist’s interpretation of the test results. Our clients appreciate the flexibility to access NeoGenomics’ medical staff for difficult or complex cases or when they are otherwise unavailable to perform professional interpretations. We believe this innovative approach to serving the needs of pathology clients’ results in longer term, more committed client relationships that are, in effect, strategic partnerships. Our extensive tech-only service offerings have differentiated us and allowed us to compete more effectively.
Global Service Offerings
We offer a comprehensive suite of technical and interpretation services to meet the needs of those clients who are not credentialed and trained in interpreting genetic tests and who require pathology specialists to interpret the testing results for them. In our global service offerings, our lab performs the technical component of the tests and our M.D.s and Ph.Ds. provide the service of interpreting the results of those tests. Our professional staff is also available for post-test consultative services. Clients using our global service offering rely on the expertise of our medical team to give them the answers they need in a timely manner to help inform their diagnoses and treatment decisions. Many of our tech-only clients also rely on our medical team for difficult or challenging cases by ordering our global testing services on a case-by-case basis. Our medical team can serve as a backup to support our clients who need help to satisfy the continued and demanding requirements of their practice. Our reporting capabilities allow for all relevant case data from our global services to be captured in one summary report. When providing global services, NeoGenomics bills for both the technical and professional component of the test, which results in a higher reimbursement level.
Client Education Programs
We believe we have one of the most extensive client education programs in the genetic and molecular testing industry. We train pathologists how to use and interpret genetic testing services so that they can better interpret technical data and render their diagnosis.
Our educational programs include an extensive library of on-demand training modules, online courses, and custom tailored on-site training programs that are designed to prepare clients to utilize our tech-only services. We offer
9
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
training and information on new cancer tests and the latest developments in the field of molecular genetic testing. Each year, we also regularly sponsor seminars and webinars on emerging topics of interest in our field. Our medical staff is involved in many aspects of our training programs.
Superior Testing Technologies and Instrumentation
We use some of the most advanced testing technologies and instrumentation in the laboratory industry. The use of next generation sequencing in our molecular testing allows us to detect multiple mutations and our proprietary techniques allow us to achieve high sensitivity in our next generation sequencing testing. In addition, we use high sensitivity Sanger sequencing, RNA and DNA quantification, SNP/Cytogenetic arrays, Fragment Length analysis, and other molecular testing technologies. Our automated FISH and Cytogenetics tools allow us to deliver the highest quality testing to our clients and our flow cytometry laboratory uses 10-color flow cytometry analysis technology on a technical-only basis. NeoGenomics is continually testing new laboratory equipment in order to remain at the forefront of new developments in the testing field.
Laboratory Information System
We believe we have a state-of-the-art LIS that interconnects our locations and provides flexible reporting solutions to clients. This system allows us to standardize testing and deliver uniform test results and images throughout our network, regardless of the location that any specific portion of a test is performed within our network. This allows us to move specimens and image analysis work between locations to better balance our workload. Our LIS also allows us to offer highly specialized and customizable reporting solutions to our tech-only clients. For instance, our tech-only FISH and flow cytometry applications allow our community-based pathologist clients to tailor individual reports to their specifications and incorporate only the images they select and then issue and sign-out such reports using our system. Our customized reporting solution also allows our clients to incorporate test results performed on ancillary tests not performed at NeoGenomics into summary report templates. This FlexREPORT feature has been well-received by clients.
National Direct Sales Force and Marketing
Our direct sales force has been trained extensively in cancer genetic testing and consultative selling skills to service the needs of clients. Our sales team for the clinical cancer testing services is organized into five regions (Northeast, Southeast, North Central, South Central and West), and we have a separate sales team for our Pharma Services division. These sales representatives utilize our custom Customer Relationship Management System (“CRM”) to manage their territories, and we have integrated all of the important customer care functionality within our LIS into the CRM so that our sales representatives can stay informed of emerging issues and opportunities within their regions. Our in-house customer care team is aligned with our field sales team to serve the needs of our clients by utilizing the same LIS and CRM. Our field teams can see in real-time when a client calls the laboratory, the reason for the call, the resolution, and if face-to-face interaction is needed for follow-up.
We continue to produce higher testing volumes and revenue due to our ongoing investment in sales and marketing. We have expanded the size of our sales team and are investing more in trade shows and in our overall marketing budget. We plan to continue to develop and execute strategic marketing plans throughout 2018.
Geographic Locations
Many high complexity laboratories within the cancer testing industry have operated a core facility on either the West Coast or the East Coast of the United States to service the needs of their customers around the country. We believe our clients and prospects desire to do business with a laboratory with national breadth and a local presence, and have developed our laboratory facility strategy accordingly. We have seven facilities, including three large laboratory locations in Fort Myers, Florida, Aliso Viejo, California, and Houston Texas. We also have four smaller laboratory locations in Fresno, California, Nashville, Tennessee, Tampa, Florida and Rolle, Switzerland. Our objective is to “operate one lab with multiple locations” in order to deliver standardized, high quality, test results. In November 2017, we opened a laboratory in Rolle, Switzerland where we are offering Pharma Services to international clients. In addition, due to growth in the Pharma Services segment, we are constructing a new, expanded laboratory in
10
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
Houston, Texas, which is a Pharma-first facility. In 2018, we are also opening a small laboratory facility in Atlanta, Georgia to offer rapid turnaround time testing to clients in that market. We intend to continue our growth and open new laboratories and/or expand our current facilities as market situations dictate and business opportunities arise.
Seasonality
The majority of our clinical testing volume is dependent on patients being treated by hematology/oncology professionals and other healthcare providers. The volume of our testing services generally declines modestly during the summer vacation season, year-end holiday periods and other major holidays, particularly when those holidays fall during the middle of the week. In addition, the volume of our testing tends to decline due to extreme adverse weather conditions, such as excessively hot or cold spells, heavy snow, hurricanes or tornados in certain regions, consequently reducing revenues and cash flows in any affected period. Therefore, comparison of the results of successive periods may not accurately reflect trends for future periods.
In our Pharma Services business, we enter into both short term and long term contracts, ranging from one month to several years. While the volume of this testing is not as directly affected by seasonality as described above, the testing volume does vary based on the terms of the contract. Many of our long term contracts contain specific performance obligations whereas the testing is performed on a specific schedule. This results in revenue that is not consistent among periods. In addition, this results in backlog that can be significant.
In the third quarter of 2017, our Houston, Texas laboratory was impacted by Hurricane Harvey and the resulting wide spread flooding in the area. While our facility was not damaged, many of our customers were unable to open for several days which impacted our business. A few weeks later our Fort Myers, Florida laboratory was impacted by Hurricane Irma. Our laboratory was not damaged and while power did go out in the area our generator kept power to our lab. Extensive power outages in the southern half of Florida did impact many of our customers who were unable to open for days after the storm had passed. The storms had a significant impact on our third quarter revenue.
Competition
For our Clinical Services segment, the genetic and molecular testing niche of the laboratory testing industry is highly competitive and, given the opportunities in this industry, we expect it to become even more competitive. Competitive factors in genetic and molecular testing generally include the reputation of the laboratory, range of services offered, pricing, convenience of sample collection and pick-up, quality of analysis and reporting, medical staff, timeliness of delivery of completed reports (i.e. turnaround times) and post-reporting follow-up for clients.
Our competitors for our Clinical Services segment in the United States are numerous and include major national medical testing laboratories, hospital laboratories and in-house physician laboratories. Our principal competitors are Quest Diagnostics and Laboratory Corporation of America. Some of our competitors have greater financial resources and production capabilities than us. These companies may succeed in developing service offerings that are more effective than any that we have or may develop, and may also prove to be more successful than we are in marketing such services. In addition, technological advances or different approaches developed by one or more of our competitors may render our service offerings obsolete, less effective or uneconomical.
We intend to continue our efforts to gain market share by offering industry-leading turnaround times, a broad service menu, high-quality test reports, new tests including proprietary ones, enhanced post-test consultation services, and the personal attention from our direct sales force. In addition, we believe our flexible reporting solutions, which enable clients to report out customized results in a secure, real-time environment, will allow us to continue to gain market share.
Our Pharma Services business competes against many other clinical research organizations and central reference laboratories. Many of these competitors are much larger and have a greater international presence than we do. Over the past year, we expanded our Pharma Services business into Europe at the request of our clients and believe that our state of the art testing menu and our high level of service along with our international expansion will allow us to continue to gain market share in this segment.
11
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
Our Pharma Services segment competitors are numerous Contract Resource Organizations or “CRO’s”. These include larger multi-national firms such as IQVIA, Covance, Parexcel and ICON. These competitors are larger than NeoGenomics and have global operations including operations in Asia, where we do not yet have service capabilities. These laboratories may be more effective than us in gaining business for global clinical trials. Many clinical reference laboratories have also entered the space in support of clinical trials and the related laboratory testing. These reference laboratories can often compete with lower pricing for smaller more limited studies. We believe our service focus and our leading Molecular and Immunohistochemistry platforms, as well as our exclusive MultiOmyx platform will continue to lead to rapid growth in this segment.
Suppliers
The Company orders its laboratory and research supplies from large national laboratory supply companies. We do not believe a short term disruption from any one of these suppliers would have a material effect on our business.
Dependence on Major Clients
We market our services to pathologists, oncologists, urologists, other clinicians, hospitals, pharmaceutical firms and other clinical laboratories throughout the United States and Europe. The Company’s client base consists of a large number of geographically dispersed clients diversified across various customer types. For the years ended December 31, 2017, 2016 and 2015, no single client accounted for more than 5% of revenue.
Payer Mix
The following table reflects our estimate of the breakdown of net clinical revenue by type of payer for the fiscal years ended December 31, 2017, 2016 and 2015:
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Medicare and other government |
|
|
15 |
% |
|
|
16 |
% |
|
|
21 |
% |
|
Commercial insurance |
|
|
18 |
% |
|
|
25 |
% |
|
|
21 |
% |
|
Client direct billing |
|
|
64 |
% |
|
|
56 |
% |
|
|
55 |
% |
|
Patient, other and year-end accruals |
|
|
3 |
% |
|
|
3 |
% |
|
|
3 |
% |
|
Total |
|
|
100 |
% |
|
|
100 |
% |
|
|
100 |
% |
Our proportion of client direct billing has increased over the years shown above, as more payers, including Medicare, private commercial insurances and Medicare Advantage plans, are practicing “consolidated payment” or “bundled payment” models where they pay the hospitals a lump sum, which is intended to include laboratory testing. This reflects an increase in the amount of risk sharing that CMS and other private payers are encouraging providers such as hospital systems to undertake. We anticipate a gradual increase in the percentage of client direct billing in the coming years.
Trademarks
The “NeoGenomics” and “Clarient” names and logos have been trademarked with the United States Patent and Trademark Office. We have also trademarked or have applications pending for the brand names NeoFISH, NeoFLOW, NeoSITE, NeoArray, NeoTYPE, NeoSCORE, NeoLAB and NeoLINK. We have also trademarked the marketing slogans, “When time matters and results count” and “Time matters, results count.”
Insurance
We maintain professional liability and numerous other insurance policies. We believe that our present insurance is sufficient to cover currently estimated exposures, but we cannot assure that we will not incur liabilities in excess of the policy coverage limits. In addition, although we believe that we will be able to continue to obtain adequate insurance coverage, we cannot assure that we will be able to do so at acceptable cost.
12
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
Our internet website address is www.neogenomics.com. Our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to section 13(a) or 15(d) of the Exchange Act are available free of charge through our website as soon as reasonably practicable after we electronically file with or furnish them to the SEC, and are available in print to any stockholder who requests a copy. Information on our website shall not be deemed incorporated into, or to be part of, this Annual Report on Form 10-K.
Additionally, the SEC maintains a website that contains reports, proxy statements, information statements and other information regarding issuers, including us, that file electronically with the SEC at www.sec.gov.
Number of Employees
As of December 31, 2017, we had approximately 1,000 full-time equivalent employees and contracted pathologists. The Company also had approximately 30 temporary contract personnel at December 31, 2017. Our employees are not represented by any union and we believe our employee relations are good.
Government Regulation
The laboratory business is subject to extensive governmental regulation at the federal, state and local levels. Our laboratories are required to be licensed by the states, certified by the federal government to participate in the Medicare and Medicaid programs, and are subject to extensive requirements as a condition of participation in various governmental health benefits programs. The failure to comply with any of the applicable federal and state laws, regulations, and reimbursement guidelines could have a material adverse effect on the Company’s business. The applicable laws and regulations, and the interpretations of them, change frequently and there can be no assurance that the Company will not be subject to audit, inquiry, or investigation with respect to some aspect of its operations. Some of the federal and state laws and regulations are described below under “Clinical Laboratory Operations,” “Anti-Fraud and Abuse Laws,” “The False Claims Act,” “Confidentiality of Health Information” and “Food and Drug Administration”.
Clinical Laboratory Operations
Licensure and Accreditation
The Company operates clinical laboratories in Florida, Tennessee, Texas and California. The laboratories are licensed as required by the states in which they are located. In addition, the laboratories in Fort Myers, Florida, Aliso Viejo, California, and Nashville, Tennessee are licensed by the State of New York as they accept clinical specimens obtained in New York. All of our laboratories are certified in accordance with the Clinical Laboratory Improvement Amendments, as amended (“CLIA”). Under CLIA, the U.S. Department of Health and Human Services (“HHS”) establishes quality standards for each category of testing performed by the laboratory. The categories of testing include waived, moderate complexity and high complexity. NeoGenomics’ laboratories are categorized as high complexity. Four of the seven site locations for NeoGenomics’ laboratories are also accredited by the College of American Pathologists (“CAP”) and actively participate in CAP’s proficiency testing programs for all tests offered by the Company. Our Tampa, Florida and Fresno, California facilities are read-only laboratories and, therefore, wouldn’t qualify for CAP accreditation. Our Houston, Texas location mainly supports Pharma Services and was recently approved for its CAP accreditation. Proficiency testing programs require the participating laboratories to test specimens that they receive from the testing entity and return the results. The testing entity, conducting an approved program, analyzes the results returned and provides to the Company a quality control report assessing the results. An important component of a quality assurance program is to establish whether the laboratory’s test results are accurate and valid.
13
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
The federal and state certification and licensure programs establish standards for the operation of clinical laboratories, including, but not limited to, qualifications of personnel and quality control. Compliance with such standards is verified by periodic inspections by inspectors employed by federal and state regulatory agencies and accrediting organizations. The Company has a Quality Assurance Committee, which is comprised of representatives of all departments of the Company, conducts routine internal surveys and requires corrective action reports in response to the findings.
Quality of Care
Our mission is to improve patient care through quality cancer genetic diagnostic services. By delivering exceptional service and innovative solutions, we aspire to become the world’s leading cancer and information company. The quality of care provided to clients and their patients is of paramount importance to us. We maintain quality control processes, including standard operating procedures, controls, performance measurement and reporting mechanisms. Our employees are committed to providing accurate, reliable and consistent services at all times. Any concerns regarding the quality of testing or services provided by the Company are immediately communicated to our Medical Team, Company management and, if necessary, the Director for Quality Systems, the Compliance Department or Human Resources Department. We also continually revise and improve our tests and work with laboratory equipment vendors to ensure that our laboratory has the highest possible quality.
Compliance Program
The health care industry is highly regulated and scrutinized with respect to fraud, abusive billing practices and improper financial relationships between health care companies and their referral sources. The Office of the Inspector General of HHS (the “OIG”) has published compliance guidance, including the Compliance Program Guidance for Clinical Laboratories in August of 1998, and advisory opinions. The Company has implemented a robust Compliance Program, which is overseen by our Board of Directors. Its objective is to ensure compliance with the myriad federal and state laws, regulations and governmental guidance applicable to our business. Our program consists of training/education of employees and monitoring and auditing Company practices. The Board of Directors has formed a Compliance Committee of the Board, which meets regularly to discuss all compliance-related issues that may affect the Company. The Company reviews its policies and procedures as new regulations and interpretations come to light to comply with applicable regulations. The Chief Compliance Officer reports directly to the Compliance Committee.
Hotline
As part of its Compliance Program, the Company provides a hotline for employees who wish to anonymously or confidentially report suspected violations of our codes of conduct, policies/procedures, or laws and regulations. Employees are strongly encouraged to report any suspected violation if they do not feel the problem can be appropriately addressed through the normal chain of command. The hotline does not replace other resources available to our employees, including supervisors, managers and human resources staff, but is an alternative channel available 24 hours a day, 365 days a year. The hotline forwards all reports to the Compliance Officer who is responsible for investigating, reporting to the Compliance Committee, and documenting the disposition of each report. The hotline forwards any calls pertaining to the financial statements or financial issues to the Chairman of the Audit Committee. The Company does not allow any retaliation against an employee who reports a compliance related issue in good faith.
Laboratory Developed Tests (“LDTs”):
The federal Food and Drug Administration (“FDA”) has regulatory responsibility over, among other areas, instruments, test kits reagents and other medical devices used by clinical laboratories to perform diagnostic testing. High complexity and CLIA-certified laboratories, such as ours, frequently develop internal testing procedures to provide diagnostic results to customers. These tests are referred to as laboratory developed tests (“LDTs”). LDTs are subject to CMS oversight through its enforcement of CLIA. The FDA has also claimed regulatory authority over all LDTs, but indicates that it has exercised enforcement discretion with regard to most LDTs offered by high
14
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
complexity CLIA-certified laboratories, and has not subjected these tests to FDA rules and regulations governing medical devices. However, the FDA has stated that it has been considering changes in the way it believes that laboratories ought to be allowed to offer these LDTs, and since 2010 publicly announced that it would be exercising regulatory authority over LDTs, using a risk-based approach that will direct more resources to tests with the highest risk of injury. In October 2014, the FDA published a draft guidance setting forth its proposed framework and timetable for regulating LTDs. The FDA received numerous comments both in support of and opposed to the draft guidance. In November 2016, FDA announced that it would not be finalizing the draft guidance. On January 13, 2017, FDA published a non-binding Discussion Paper to “advance the public discussion by providing a possible approach to spur further dialogue.” The Discussion Paper sets forth a possible LDT regulatory approach where LDTs currently on the market would be exempt from FDA regulation except for adverse event and malfunction reporting, and regulation of new and modified LDTs would be phased in over four years, based on risk. It remains uncertain whether FDA’s proposed approach will be adopted by the FDA or Congress. It is also uncertain what position the new administration will adopt with respect to LDTs. It is possible that the FDA could adopt a new policy, or Congress could enact new legislation, that may result in increased regulatory burdens for us to register and continue to offer our tests or to develop and introduce new tests, or modify existing tests and may increase our costs. We cannot be certain as to which of our tests would require FDA review and approval, and if approval was to be required, that our tests could obtain FDA approval.
The federal laws governing Medicare, Medicaid and other federal health benefits, as well as other state and federal laws, regulate certain aspects of the relationships between health care providers, including clinical laboratories, and their referral sources, including physicians, hospitals, other laboratories and other entities. We are subject to the federal Anti-Kickback Statute (“federal AKS”), as well as similar state statutes and regulations, which prohibit the offer, payment, solicitation or receipt of any form of remuneration in return for referring, ordering, leasing, purchasing or arranging for or recommending the ordering, purchasing or leasing of items or services payable by Medicare, Medicaid or any other federally funded healthcare program. The federal AKS defines remuneration to include anything of value, in cash or in kind, and thus can implicate financial relationships including payments not commensurate with fair market value, such as in the form of space, equipment leases, professional or technical services or anything else of value.
The federal AKS is an “intent‑based” statute, meaning that a violation occurs when one or both parties intend the remuneration to be in exchange for or to induce referrals. Violations of the federal AKS may result in substantial civil or criminal penalties, including criminal fines of up to $25,000, imprisonment of up to five years, civil penalties under the federal CMP Law of up to $50,000 for each violation, plus three times the remuneration involved, civil penalties under the federal False Claims Act of up to $11,000 for each claim submitted, plus three times the amounts paid for such claims and exclusion from participation in the Medicare and Medicaid programs.
Because of the broad proscriptions of the federal AKS, subsequent federal law required the HHS to publish regulations to guide the health care community in structuring relationships that would not violate the law. The OIG published regulations outlining certain categories of relationships between health care providers and persons or entities that may have a referral relationship that would be deemed not to violate the federal AKS. These regulations are known as the Safe Harbor Regulations (the “Safe Harbor Regulations”) because persons who enter into transactions that comply with all of the criteria for an applicable safe harbor will not violate the AKS. The Safe Harbor Regulations are narrowly drafted to avoid inadvertently immunizing prohibited conduct. A relationship or transaction that does not meet all of the criteria of an applicable Safe Harbor Regulation is not deemed to be illegal per se, rather it may be subject to additional scrutiny. The Company endeavors to comply with the Safe Harbor Regulations, but there can be no assurance that the Company would not be subject to investigation and, if investigated, that relationships could be found not to comply with the Safe Harbor Regulations.
Further, most states have adopted similar anti-kickback laws prohibiting the offer, payment, solicitation or receipt of remuneration in exchange for referrals, and typically impose criminal and civil penalties as well as loss of licenses. Some of these state laws apply to items and services paid for by private payers as well as to government payers. In addition, many states have adopted laws prohibiting the splitting or sharing of fees between physicians and non‑physicians, as well as between treating physicians and referral sources. We believe our arrangements with physicians comply with the federal AKS, and state anti-kickback and fee‑splitting laws of the states in which we operate, however, if government regulatory authorities were to disagree, we could be subject to civil and criminal
15
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
penalties, and be required to restructure or terminate our contractual and other arrangements with physicians. This could result in a loss of revenue and have a material adverse effect on our business.
Medicare Payment Guidelines
We have various billing arrangements with our clients and with third party payers, including the Medicare program. When the Company bills the client for all, or a portion of, a lab test performed, these client billing arrangements are priced competitively at fair market value. These client billing arrangements may implicate the prohibition of the Medicare program against charging the Medicare or Medicaid programs fees substantially in excess of the Company’s usual and customary charges. These billing arrangements may also implicate the federal Stark Law and the federal and state anti-kickback statutes.
Federal law authorizes the Secretary of HHS to suspend or exclude providers from participation in the Medicare and Medicaid programs if providers charge Medicare or state Medicaid programs fees “substantially in excess” of their “usual charges.” The OIG has stated in commentary to various final and proposed regulations its position that this statute has limited applicability to the current Medicare reimbursement system, though the OIG has also commented “we note that ancillary services, such as laboratory tests and drugs, would remain subject to these regulations, even when furnished by physicians.” [F.R., Vol. 68, No. 178, September 15, 2003 at 53940]. As such, application of this prohibition to the Company’s business is not clear, but the government could scrutinize the Company’s pricing and billing arrangements and determine to apply this law.
The Centers for Medicare and Medicaid Services promulgated, in 2009, a revision to the regulation that prohibits the mark up of purchased diagnostic services [42 C.F.R. §414.50] (the “Anti-Markup Rule”). The Anti-Markup Rule prohibits a physician or other supplier from marking up the price paid for the technical or professional component of a diagnostic test that was ordered by the billing physician or supplier and which was performed by a physician who does not share a practice with the billing physician or supplier. The billing physician is prohibited from billing the Medicare program an amount greater than the lesser of: (i) the performing supplier’s net charge to the billing physician; (ii) the billing physician’s actual charge; or (iii) the fee schedule amount for the test that would be allowed if the performing supplier billed directly.
In light of the various federal regulations and guidance from the OIG, the Company seeks to price its products competitively while endeavoring to meet applicable statutes and regulations.
Physician Self-Referral Laws
The federal law referred to as the “Stark Law”, named after U.S. Representative Fortney “Pete” Stark, prohibits physicians who have a financial relationship with an entity from referring Medicare and Medicaid patients to that entity for the provision of designated health services unless the transaction meets an exception to the law. A “financial relationship” includes both an ownership interest and/or a compensation arrangement with a physician, both direct and indirect, and DHS includes, but is not limited to, laboratory services.
The Stark Law prohibits an entity that receives a prohibited DHS referral from seeking payment from Medicare and Medicaid for any DHS services performed as a result of such a referral, unless an arrangement is carefully structure to satisfy every requirement of a regulatory exception. The Stark Law is a strict liability statute, and thus any technical violation requires repayment of all “tainted” referrals, regardless of the intent. Penalties for violating the Stark Law may include the denial of payment to an entity for the impermissible provision of DHS, the requirement to refund any amounts collected in violation of the Stark Law, and civil monetary penalties of up to $15,000 for each violation and $100,000 for each circumvention arrangement or scheme. Other implications of a Stark Law violation may include criminal penalties, exclusion from Medicare and Medicaid programs, and potential False Claims Act liability, including via “qui tam” action. The Company endeavors to structure its financial relationships in compliance with the Stark Law and with similar state physician self-referral laws.
Further, many states have promulgated self‑referral laws and regulations similar to the federal Stark Law, but these vary significantly based on the state. For example, the Florida Patient Self-Referral Act of 1992, as amended, (the “Florida Self-Referral Act”) is similar to the Stark law, but is narrower in some respects and broader in others. In
16
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
addition to services reimbursed by Medicaid or government payers, often these state laws and regulations can encompass services reimbursed by private payers as well. Penalties for violating state self-referral laws and regulations vary based on the state, but often include civil and criminal penalties, exclusion from Medicaid, and loss of licenses. Our financial arrangements with physicians are governed by the federal Stark Law and similar state self-referral laws, and we rely on certain exceptions to the Stark Law with respect to such relationships. While we believe that our financial relationships with physicians and referral practices are in compliance with applicable laws and regulations, we cannot guarantee that government authorities would agree. If we are found by the government to be in violation of the Stark Law or a similar state self-referral law, we could be subject to significant penalties, including fines as specified above, exclusion from participation in government and private payer programs and requirements to refund amounts previously received from government.
The False Claims Act
The federal False Claims Act prohibits any person or entity from knowingly presenting, or causing to be presented, to the U.S. government, or to a Medicare program contractor, a false or fraudulent claim for payment, or knowingly making or using a false record or statement to have a false claim paid by the government, or conspiring to defraud the U.S. government, or knowingly making or using a false statement to conceal an obligation to pay the government, or improperly retaining overpayments from, the government. A violation of the federal False Claims Act is punishable by a civil penalty of $5,500 to $11,000 for each separate false claim plus three times the amount of damages sustained by the government. Further, False Claims Act liability may lead to exclusion from participation in Medicare, Medicaid and other federal healthcare programs. The False Claims Act’s “whistleblower” or “qui tam” provisions are being used with more frequency to challenge the reimbursement practices of providers and suppliers. Those provisions allow a private individual to bring an action on behalf of the government alleging that the defendant has submitted false claims for payment to the federal government. The government must decide whether to intervene in the lawsuit and whether to prosecute the case. If it declines to do so, the individual may pursue the case alone, although the government must be kept apprised of the progress of the lawsuit. Whether or not the federal government intervenes in the case, it will receive the majority of any recovery. The successful qui tam relator who brought the case is entitled to a portion of the proceeds and its attorneys’ fees and costs. As most qui tam cases are filed by current or former employees, an effective compliance program plays a crucial role in reducing the Company’s exposure to liability. It is also a criminal offense, under Title 18 U.S. Code, Section 287, for a person or entity to make a claim against the United States or any department or agency, knowing the claim to be false, fictitious or fraudulent. The penalty is a fine, and imprisonment of up to five years. The federal False Claims Act has been an effective enforcement tool for the federal government. Many states have enacted similar false claims acts as well.
The Company seeks to structure its arrangements with physicians and other clients to be in compliance with the Anti-Kickback Statute, Stark Law, state laws, and the federal False Claims Act and to stay abreast of current developments and changes in the law and regulations. However, these laws and regulations are complex and subject to interpretation. Consequently, we are unable to ascertain with certainty that any of our transactions will not be subject to scrutiny and, if scrutinized, will not result in sanctions or penalties. The Company has taken, and will continue to take, actions to endeavor to ensure compliance with the myriad federal and state laws that govern our business.
Confidentiality and Security of Personal Health Information
The Health Insurance Portability and Accountability Act of 1996, as amended (“HIPAA”), contains provisions that protect individually identifiable health information from unauthorized use or disclosure by covered entities and their business associates. The Office for Civil Rights of HHS, the agency responsible for enforcing HIPAA, has published regulations to address the privacy (the “Privacy Rule”) and security (the “Security Rule”) of protected health information (“PHI”). The Company is a covered entity under HIPAA and has adopted policies and procedures to comply with the Privacy Rule and the Security Rule and HIPAA statute. The health care facilities and providers that refer specimens to the Company are also bound by HIPAA. HIPAA also requires that all providers who transmit claims for health care goods or services electronically utilize standard transaction and data sets and to standardize national provider identification codes. The Company has taken necessary steps to comply with HIPAA regulations,
17
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
utilizes standard transaction data sets, and has obtained and implemented national provider identifiers, or NPIs, as the standard unique health identifier in filing and processing health care claims and other transactions.
The American Recovery and Reinvestment Act (“ARRA”) recently enacted the HITECH Act which extends the scope of HIPAA to permit enforcement against business associates for a violation, establishes new requirements to notify the Office for Civil Rights of HHS of a breach of HIPAA, and allows the Attorneys General of the states to bring actions to enforce violations of HIPAA. Rules implementing various aspects of HIPAA are continuing to be promulgated. With respect to these rules, commencing July 1, 2012, CMS required all HIPAA-covered entities such as the Company to conduct electronic claim submissions and related electronic transactions under a new HIPAA transaction standard called Version 5010.
In addition to the HIPAA Privacy Rule and Security Rule described above, the Company is subject to state laws regarding the handling and disclosure of patient records and patient health information. These laws vary widely. Penalties for violation include sanctions against a laboratory’s licensure as well as civil or criminal penalties. Additionally, private individuals may have a right of action against the Company for a violation of a state’s privacy laws. We believe we are in material compliance with current state laws regarding the confidentiality of health information and will continue to monitor and comply with new or changing state laws.
The Fair and Accurate Credit Transactions Act of 2003, enacted on Dec. 4, 2003, directed the Federal Trade Commission to implement regulations to protect consumers against identity theft. The Federal Trade Commission issued what are referred to as the “Red Flag Rules”, but the effective date for enforcement has been delayed several times. The Red Flag Rules are now subject to enforcement as of January 1, 2012. The Red Flag Program Clarification Act of 2010 (“RFPCA”) gave some relief to health care providers by changing the definition of “creditor”, thereby narrowing the application to health care providers who do not otherwise obtain or use consumer reports or furnish information to consumer reporting agencies in connection with a credit transaction. Health care providers who act as a “creditor” to any of its patients with respect to a “covered account” are required to implement an identity theft protection program to safeguard patient information. A creditor includes any entity that regularly in the course of business obtains or uses consumer reports in connection with credit transactions, furnishes information to a consumer reporting agency in connection with a credit transaction, or advances funds to or on behalf of a person based on the person’s obligation to repay the funds or repayable from specific property pledged by or on behalf of the person. But, a creditor, as defined in the RFPCA, that advances funds on behalf of a person for expenses incidental to a services provided by the creditor to that person is not subject to the Red Flag Rules. The Company has developed a written program designed to identify and detect the relevant warning signs – or “red flags” – of identity theft and establish appropriate responses to prevent and mitigate identity theft in order to comply with the Red Flag Rules. We are also developing a plan to update the program, and the program will be managed by senior management staff under the policy direction of our Board of Directors. The Company intends to take such steps as necessary to determine the extent to which the Red Flag Rules apply to it and to take such steps as necessary to comply.
18
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
Executive Officers of the Company
The following table sets forth certain information regarding members of the Board of Directors and our executive officers as of March 1, 2018:
|
Name |
|
Age |
|
Position |
|
Board of Directors: |
|
|
|
|
|
Douglas M. VanOort |
|
62 |
|
Chairman of the Board of Directors and Chief Executive Officer |
|
Steven C. Jones |
|
54 |
|
Executive Vice President, Chief Compliance Officer, Board Member |
|
Kevin C. Johnson |
|
63 |
|
Board Member |
|
Raymond R. Hipp |
|
75 |
|
Board Member |
|
Bruce K. Crowther |
|
66 |
|
Board Member |
|
William J. Robison |
|
82 |
|
Board Member |
|
Lynn A. Tetrault |
|
55 |
|
Board Member |
|
Alison L. Hannah |
|
57 |
|
Board Member |
|
Stephen Kanovsky |
|
55 |
|
Board Member |
|
Other Executives: |
|
|
|
|
|
George A. Cardoza |
|
56 |
|
Senior Vice President, Chief Financial Officer |
|
Dr. Maher Albitar |
|
62 |
|
Senior Vice President, Chief Medical Officer and Director of Research & Development |
|
Dr. Steven Brodie |
|
57 |
|
Vice President of Operations |
|
Robert J. Shovlin |
|
47 |
|
President, Clinical Services Division |
|
Steven A. Ross |
|
53 |
|
Vice President, Chief Information Officer |
|
Jennifer M. Balliet |
|
40 |
|
Vice President, Chief Culture Officer |
|
Kathryn B. McKenzie |
|
33 |
|
Principal Accounting Officer and Vice President of Finance |
Members of the Company’s Board of Directors are elected at the annual meeting of stockholders and hold office until their successors are elected. The Company’s officers are appointed by the Board of Directors and serve until their resignation or removal by the Board and are subject to employment agreements, if any, approved and ratified by the Board. There are no family relationships between any of our officers or directors.
In addition, pursuant to the Investor Board Rights, Lockup and Standstill Agreement dated December 30, 2015, GE Medical Systems has the right to designate one individual for approval and we are required to appoint such designee, as a director to our Board of Directors. Kieran Murphy, President and Chief Executive Officer of GE Healthcare Life Sciences was appointed to the Board pursuant to such agreement. In 2017, Kieran Murphy was appointed President and Chief Executive Officer of GE Healthcare, a business unit of General Electric and resigned his role on the Board. Stephen Kanovsky was appointed to serve as a member of the Board effective immediately to fill the vacancy created by Mr. Murphy’s resignation.
Douglas M. VanOort, – Chairman of the Board of Directors and Chief Executive Officer
Mr. VanOort has served as the Chairman of the Board of Directors and Chief Executive Officer of NeoGenomics since October 28, 2009. For seven months prior to October 2009, he served as Chairman of the Board of Directors, Executive Chairman and Interim Chief Executive Officer. Prior to joining NeoGenomics, Mr. VanOort was a General Partner with a private equity firm, and a Founding Managing Partner of a venture capital firm. From 1982 through 1999, Mr. VanOort served in various positions at Corning Incorporated and at its spin-off company, Quest Diagnostics, Inc. During the period from 1995 through 1999, he served as the Senior Vice President Operations for Quest Diagnostics, Inc. which was then a $1.5 billion newly formed NYSE-traded Company. During the period of 1989 to 1995, he held senior executive positions at Corning Life Sciences, Inc., including Executive Vice President. Corning Life Sciences Inc. had revenues of approximately $2 billion and was spun-off in a public transaction to create both Quest Diagnostics and Covance, Inc. From 1982 to 1989, Mr. VanOort served in various executive positions at Corning Incorporated, including Director of Mergers & Acquisitions. Mr. VanOort currently serves as a member of the Board of Directors of several privately-held companies, and is a principal owner of a privately-held retail hardware store chain. Mr. VanOort is a graduate of Bentley University.
19
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
Steven C. Jones – Executive Vice President, Chief Compliance Officer, Board Member
Mr. Jones served as a director since October 2003, as Executive Vice President since November 4, 2016, and as Chief Compliance Officer since February 7, 2013. Mr. Jones served as Chief Financial Officer for the Company from October 2003 until November 30, 2009, and was Executive Vice President – Finance from November 30, 2009 to November 4, 2016. Mr. Jones is also the founder and Chairman of the Aspen Capital Group, a private equity investment firm, and has been President and Managing Director of Aspen Capital Advisors since January 2001. Prior to that Mr. Jones was a chief financial officer at various public and private companies and was a Vice President in the Investment Banking Group at Merrill Lynch & Co. Mr. Jones received his B.S. degree in Computer Engineering from the University of Michigan in 1985 and his MBA degree from the Wharton School of the University of Pennsylvania in 1991. He also serves as Chairman of the Board of T3 Communications, Inc. and he is a member of the Board of XG Sciences, Inc. and ERP Maestro, Inc.
Kevin C. Johnson – Board Member
Mr. Johnson has served as a director since 2010. Mr. Johnson was the Chief Executive Officer for United Allergy Services, a provider of allergy testing and immunotherapy services, from September 2014 through July 2015. From January 2003 until September 2014 Mr. Johnson was retired. From May 1996 until January 2003, Mr. Johnson was Chairman, Chief Executive Officer and President of DIANON Systems, Inc., a publicly-traded cancer diagnostic services company providing anatomic pathology and molecular genetic testing services to physicians nationwide. During that time, DIANON grew annual revenues from approximately $56 million in 1996 to approximately $200 million in 2002. DIANON was sold to Laboratory Corporation of America (NYSE: LH) in January of 2003. Prior to joining DIANON in 1996, Mr. Johnson was employed by Quest Diagnostics and Quest’s predecessor, the Life Sciences Division of Corning, Incorporated, for 18 years, and held numerous management and executive level positions.
Raymond R. Hipp – Board Member
Mr. Hipp has served as a director since February 2011. Mr. Hipp is a retired senior executive that has been involved in consulting work over the last few years involving mergers and acquisitions as well as serving on the Board of Directors for several public companies. From July 1998 until his retirement in June 2002, Mr. Hipp served as Chairman, President and CEO of Alternative Resources Corporation, a provider of information technology outsourcing services. From August 1996 until May 1998, Mr. Hipp was the Chief Executive Officer of ITI Marketing Services, a provider of marketing services. Prior to that, Mr. Hipp held senior executive positions with several other firms. Mr. Hipp has a B.S. from Southeast Missouri State University. Mr. Hipp served on the Board of Directors and on the Audit Committee of Gardner Denver, Inc. (NYSE: GDI), an industrial manufacturing company, for over 14 years.
Bruce K. Crowther – Board Member
Mr. Crowther has served as a Director since October 2014. Mr. Crowther retired in 2013 as President and Chief Executive Officer of Northwest Community Healthcare where he served for 23 years. Northwest Community Healthcare is an award winning hospital offering a complete system of care. Mr. Crowther has a B.S. in Biology and an M.B.A. from Virginia Commonwealth University. Mr. Crowther serves on the Board of Directors of Wintrust Financial Corporation, a public company and serves on the Board of Directors of Barrington Bank and Trust which is a Wintrust Financial Corporation owned Company. He was previously the Chairman and currently a Director of the Max McGraw Wildlife Foundation; a not for profit organization committed to conservation education and research.
William J. Robison – Board Member
Mr. Robison has served as a director since May 2007. Mr. Robison, who is retired, spent his entire 41 year career with Pfizer, Inc. At Pfizer, he rose through the ranks of the sales organization and became Senior Vice President of Pfizer Labs in 1986. In 1990, he became General Manager of Pratt Pharmaceuticals, a then new division of the U.S. Pharmaceuticals Group, and in 1992 he became the President of the Consumer Health Care Group. In 1996 he
20
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
became a member of Pfizer’s Corporate Management Committee and was promoted to the position of Executive Vice President and head of Worldwide Corporate Employee Resources. Mr. Robison retired from Pfizer in 2001. Mr. Robison was previously a board member of the University of Louisiana – Monroe, MWI Veterinary Supply Company, Inc., USO of Metropolitan New York, Inc., the Human Resources Roundtable Group, the Pharmaceutical Human Resource Council, the Personnel Round Table, and the Employee Relations Steering Committee for The Business Round Table. Mr. Robison was also a founding member of the Marine Corps Museum.
Lynn A. Tetrault – Board Member
Ms. Tetrault has served as a director since June 2015. Ms. Tetrault is founder and principal of Anahata Leadership, an advisory firm focused on supporting the leadership effectiveness and development of executive women. She worked from 1993 to 2014 with AstraZeneca, PLC most recently as Executive Vice President Human Resources and Corporate Affairs. Ms. Tetrault was responsible for all human resources strategy, talent management, executive compensation and related activities, internal and external communications, government affairs, corporate reputation and corporate social responsibility for the Company. Ms. Tetrault has an undergraduate degree from Princeton University and a J.D. from the University of Virginia Law School.
Alison L. Hannah – Board Member
Dr. Hannah has served as a director since June 2015. Dr. Hannah has over 25 years' experience in the development of investigational cancer chemotherapies. Since 2000, she has served as a consultant to the pharmaceutical industry, working with over 20 companies with a focus on molecularly targeted therapy. Prior to this, she worked as Senior Medical Director at SUGEN on various compounds, including Sutent approved in kidney cancer, and Quintiles, a global Contract Research Organization. Dr. Hannah specializes in clinical development strategy, and has filed over 30 Investigational New Drug applications for new molecular entities and 7 New Drug Applications. She participates in Data Monitoring Committees, Scientific Advisory Boards and Independent Review Committees for clinical trials. She has a bachelor's degree in biochemistry and immunology from Harvard University and her medical degree from the University of Saint Andrews. She is a member of ASCO, AACR, ASH, ESMO and a Fellow with the Royal Society of Medicine.
Stephen Kanovsky – Board Member
Mr. Kanovsky is General Counsel, Global Innovation of GE Healthcare, a business unit of General Electric that provides medical technologies and solutions to the global healthcare industry and supports customers in over 100 countries with a broad range of services and systems, from diagnostic imaging and healthcare IT through to molecular diagnostics and life sciences. Mr. Kanovsky has over 23 years of legal experience in the global life sciences and biotechnology industry. Mr. Kanovsky earned his bachelor’s degree in 1984 from the University of Pennsylvania. He subsequently graduated from Temple University’s School of Pharmacy with a master’s degree in Pharmacology and Temple University’s School of Law with a juris doctorate degree. Mr. Kanovsky also holds a master’s degree in business administration from Saint Joseph’s University’s Haub School of Business.
George A. Cardoza – Senior Vice President, Chief Financial Officer
Mr. Cardoza has served as Chief Financial Officer since November 2009. Prior to that from March 2008 to November 2009, Mr. Cardoza served as the Chief Financial Officer of Protocol Global Solutions, Inc., a privately held international marketing company. Mr. Cardoza also served as the Controller of Protocol Global Solutions from March 2006 to March 2008. From April 1991 to March 2006, Mr. Cardoza was employed by Quest Diagnostics Inc., a diagnostic testing, information and services company, in a number of positions, including the position of Controller—Central Region from 2001 to March 2006. At Quest Mr. Cardoza was responsible for overseeing all the financial operations of the Central Region, which had revenue of over $1.2 billion in 2006. Prior to his time with Quest, he worked for Sony Music Entertainment Inc. and the Continental Grain Company in various financial roles. Mr. Cardoza received his B.S. from Syracuse University in finance and accounting and has received his M.B.A. from Michigan State University.
21
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
Maher Albitar, M.D. – Senior Vice President, Chief Medical Officer and Director of Research and Development
Dr. Albitar has served as Chief Medical Officer and Director of Research and Development since January 2012. From 2008 to 2011, Dr. Albitar served as the Medical Director for Hematopathology and Oncology, Nichols Institute of Quest Diagnostics, and Chief R&D Director for Hematopathology and Oncology for Quest Diagnostics, a diagnostic testing, information and services company. From 2003 to 2008, Dr. Albitar served as the Director of Hematopathology for the Nichols Institute of Quest Diagnostics. From 2005 to 2011, Dr. Albitar also served as a Board member of Associated Diagnostics Pathologists, Inc. From 1991 to 2003, Dr. Albitar held various faculty positions at The University of Texas MD Anderson Cancer Center. Dr. Albitar previously served as the Chief Medical Officer of Health Discovery Corporation (“HDC”) and a member of the Board of Directors of HDC. Dr. Albitar has also served as a consultant to multiple companies. Dr. Albitar received his medical degree in 1979 from Damascus Medical School in Damascus, Syria. Dr. Albitar has co-authored approximately 300 peer reviewed articles, chapters and reviews.
Steven G. Brodie, Ph.D. – President, Pharma Services Division
Dr. Brodie has served as the President of our Pharma Services Division since September, 2016. Prior to this he had served as Chief Scientific Officer of NeoGenomics since April 2015. Dr. Brodie is also the Laboratory Director for our Fort Myers, FL lab facility, a role he has held since 2014. He also has served as our Director of Molecular Genetics and Cytogenetics since 2011. Prior to joining NeoGenomics, Dr. Brodie served as a Senior Director of Cytogenetics, Assistant Director of Molecular Genetics, and Scientific Director of Maternal Serum Screening at Quest Diagnostics (Specialty Laboratories) in Valencia Ca. In addition to his clinical responsibilities, he trained Pathology residents in genetic testing for Loma Linda University Medical Center as the Affiliate Rotation Director and the University of Southern California, Keck SOM as a Clinical Assistant Professor of Pathology. Prior to joining Quest Diagnostics, he held a variety of research and clinical positions at the National Institutes of Health, University of New Mexico School of Medicine, and the University of California Los Angeles David Geffen School Of Medicine. Dr. Brodie was trained in Genetics at the University of California Los Angeles/Cedar-Sinai Medical Center medical genetics training program. He received a Ph.D. in Biomedical Sciences from the University of New Mexico School of Medicine and Clinical Molecular Genetics and Cytogenetics training at the University of California Los Angeles. Dr. Brodie is Board Certified by the American Board of Medical Genetics and Genomics and holds Directors Licenses in California, Florida, Tennessee, and New York.
Robert J. Shovlin – President, Clinical Services Division
Mr. Shovlin has served as the President of our Clinical Services Division since September, 2016. Prior to this, he had served as our Chief Growth Officer since the acquisition of Clarient Inc. (“Clarient”) in 2015. From his hire date in October 2014 until the Clarient acquisition, Mr. Shovlin served as the Chief Operating Officer of NeoGenomics. From 2012 until October 2014, Mr. Shovlin served as Chief Development officer for Bostwick Laboratories, a provider of anatomic pathology testing services targeting urologists and other clinicians, where he was responsible for Sales, Marketing, Managed Care, Business Development, and Clinical Trials. From 2005 until 2011, he served in progressively more responsible positions, including President and Chief Executive Officer, for Aureon Biosciences, Inc., a venture-backed diagnostics company focused on developing novel and proprietary prostate cancer tests. Mr. Shovlin also served as Executive Director for Anatomic Pathology and Director of Managed Care for Quest Diagnostics from 2003 until 2005, and held sales leadership positions at Dianon Systems from 1997 until 2003. Mr. Shovlin served as a Captain, Infantry Officer in the United States Marine Corps from 1992 until 1997 where he served as a Platoon and Company Commander with 1st Battalion 4th Marines and as an Instructor and Staff Platoon Commander at the Basic School. He holds a Bachelor of Science Degree from Pennsylvania State University, and a Masters of Business Administration from Rutgers University.
Steven A. Ross – Vice President, Chief Information Officer
Mr. Ross has served as Chief Information Officer since April 2013. Prior to joining the Company, Mr. Ross served as Vice President Technology at Chico’s FAS, Inc. during the period from 2003 to 2013 where he participated in the direction of all information technology resource planning, budgeting, technology associate development coaching and operation initiatives for the $2.5 billion dollar global consumer products company. Prior to that Mr. Ross
22
NEOGENOMICS, INC.
ITEM 1. BUSINESS (CONTINUED)
worked for Zinn Corporation as a Project Director, assisting Target Inc. Mr. Ross has his Bachelor of Science from New Mexico State University.
Jennifer M. Balliet – Vice President, Chief Culture Officer
Ms. Balliet has served as our Chief Culture Officer since September, 2016. Prior to that, she had served as our Vice President of Human Resources since April 2015. Ms. Balliet joined NeoGenomics in 2008 and has steadily increased her responsibilities and was previously serving as Director of Human Resources. During her time with NeoGenomics, she managed the Human Resources process as the Company grew from 100 employees to approximately 1,000 employees. As Vice President of Human Resources, Ms. Balliet has responsibility for all areas of our Human Resources including recruiting, training, development, compensation, incentive plans and organizational development. Ms. Balliet received her B.S. degree in Psychology and M.S. degree in Business Management from the University of Florida.
Kathryn B. McKenzie – Principal Accounting Officer and Vice President of Finance
Ms. McKenzie has served as our Principal Accounting Officer and Vice President of Finance since October 2017. Prior to joining the Company, Ms. McKenzie served at Chico’s FAS, Inc. in various roles including Assistant Controller and Director of Financial Reporting and Treasury. Ms. McKenzie also previously served as Audit Manager for Ernst and Young. Ms. McKenzie is a Certified Public Accountant and holds a Master’s of Science in Accountancy from the University of North Carolina Wilmington.
23
NEOGENOMICS, INC
ITEM 1A. RISK FACTORS
We are subject to various risks that may materially harm our business, financial condition and results of operations. They are not, however, the only risks we face. Additional risks and uncertainties not presently known to us or that we currently believe not to be material may also adversely affect our business, financial condition or results of operations. An investor should carefully consider the risks and uncertainties described below and the other information in this filing before deciding to purchase our common stock. If any of these risks or uncertainties actually occurs, our business, financial condition or operating results could be materially harmed. In that case, the trading price of our common stock could decline or we may be forced to cease operations.
Risks Relating to Our Business
Our business is subject to rapid scientific change, which could have a material adverse effect on our business, results of operations and financial condition.
The market for genetic and molecular testing services is characterized by rapid scientific developments, evolving industry standards and customer demands, and frequent new product introductions and enhancements. For example, new tests developed by our competitors may prove superior and replace our existing tests. Our future success will depend in significant part on our ability to continually improve our offerings in response to both evolving demands of the marketplace and competitive service offerings, and we may be unsuccessful in doing so which could have a material adverse effect on our business, results of operations and financial condition. Certain technological changes such as advances in point-of-care testing, could reduce the need for the laboratory tests we provide.
The market for our services is highly competitive, which could have a material adverse effect on our business, results of operations and financial condition.
The market for genetic and molecular testing services is highly competitive and we expect competition to continue to increase. We compete with other commercial clinical laboratories in addition to the in-house laboratories of many major hospitals and physician practices. Many of our existing competitors have significantly greater financial, human, technical and marketing resources than we do. Some physician groups and hospitals have decided to internalize testing rather than use an outsourced laboratory such as our Company. Our competitors may develop products and services that are superior to ours or that achieve greater market acceptance than our offerings. We may not be able to compete successfully against current and future sources of competition and in such cases, this may have a material adverse effect on our business, results of operations and financial condition.
Proposed government regulation of LDTs may result in delays to launching certain laboratory tests and increase our costs to implement new tests.
We frequently develop testing procedures to provide diagnostic results to clients that cannot currently be provided using test kits approved or cleared by the FDA. The FDA has been considering changes to the way that it regulates these LDTs. Currently all LDTs are conducted and offered in accordance with the CLIA, and individual state licensing procedures. The FDA has published a draft guidance document that would require FDA clearance or approval of a subset of LDTs, as well as a modified approach for some lower risk LDTs that may require FDA oversight short of the full premarket approval or clearance process. Congress may enact legislation to provide a regulatory framework for the FDA’s role with regard to LDTs. As a result, there is a risk that the FDA’s proposed regulatory process could delay the offering of certain tests and result in additional validation costs and fees. There is also an associated risk for us that some tests currently offered might become subject to FDA premarket approval or clearance. This FDA approval or clearance process may be time-consuming and costly, with no guarantee of ultimate approval or clearance.
On July 31, 2014 the FDA issued a notification to Congress of the “Anticipated Details of the Draft Guidance for Industry, Food and Drug Administration Staff, and Clinical Laboratories: Framework for Regulatory Oversight of Laboratory Developed Tests,” or the Draft LDT Guidance. As described in this notification, the FDA planned to provide draft guidance to clinical laboratories that develop their own LDTs regarding how the FDA intends to regulate such laboratories under the Federal Food, Drug, and Cosmetic Act. On October 3, 2014 the FDA issued the draft guidance to clinical laboratories. The regulatory framework will use a risk-based approach to enforce the FDA’s premarket review requirements, and for high-risk tests, the framework may require laboratories to use FDA-
24
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
approved tests, if available, rather than LDTs. If implemented, the framework outlined in the Draft LDT Guidance may also require us to obtain premarket clearance or approval for certain of our LDTs. Implementation of this framework would include a lengthy phase-in period ranging from two to nine years depending on the risk assessment rating of each particular test. The FDA provided an opportunity for public comment through February 2015 and received numerous public comments in response to the Draft LDT Guidance. In January 2017 the FDA announced that it would not issue a final guidance on the oversight of LDTs at the request of various stakeholders to allow for further public discussion on an appropriate oversight approach, and to give congressional authorizing committees the opportunity to develop a legislative solution. At the same time, Congress, the FDA, and various industry stakeholders have worked to provide recommendations for comprehensive reform of LDAs. Recently, Congress has submitted a legislative discussion draft, the Diagnostic Accuracy and Innovation Act (“DAIA”) to the FDA and requested technical assistance on the draft. However, it remains unknown whether the regulatory framework ultimately implemented by the FDA will differ substantially from the framework described in the Draft LDT Guidance or in the DAIA. This FDA regulation may result in increased regulatory burdens for us to register and continue to offer our tests or to develop and introduce new tests and may increase our costs. We do not yet know which of our tests would be classified as high-risk and would require a full FDA approval. If such approval was required, we cannot be certain that our tests would obtain FDA approval or clearance.
In the event that, in the future, the FDA and/or congressional authorizing committees begin to regulate our tests, it could require a significant volume of applications with the FDA and/or document responses to congressional authorizing committees which would be burdensome and the FDA and/or congressional authorizing committees could take a long time to review such applications and/or document responses if every lab in the country files a large volume of applications and/or document responses for each of their LDTs.
In November of 2017, CMS initiated a national coverage analysis for the use of Next Generation Sequencing “NGS” diagnostic tests for patients with advanced cancer. The proposed decision memo was released and open to a public comment period. Through this national coverage analysis, CMS is considering making changes to reimbursement for NGS testing which once finalized could directly affect our revenue for this test type.
Healthcare reform programs may impact our business and the pricing we receive for our services.
In March of 2010, health care reform legislation known as the “Patient Protection and Affordable Care Act,” also known as the ACA, was passed into law. The ACA also makes changes that are expected to significantly impact the pharmaceutical and medical device industries and clinical laboratories. For example, effective December 31, 2017, each medical device manufacturer must pay sales tax in an amount equal to 2.3% of the price for which such manufacturer sells its medical devices that are listed with the FDA. Although the FDA issued Draft LDT Guidance that, if finalized, would regulate certain clinical laboratory tests that are developed and validated by a laboratory for its own use, or LDTs, as medical devices, none of our LDTs such as our prostate cancer test are currently listed with the FDA. We cannot assure you that the tax will not apply to services such as ours in the future.
The ACA contains several provisions that seek to limit Medicare spending in the future. One key provision in the ACA is the establishment of “Accountable Care Organizations,” or ACOs, under which hospitals and physicians are able to share savings that result from cost control efforts. We cannot predict how the continued establishment and implementation of these new business models will impact our business. There is the possibility that these organizations will seek to lower reimbursement for the services we provide and some may potentially restrict access to our services. We may not be able to gain access into certain ACOs. These changes could have an adverse and material impact on our operations. In furtherance of health care reform and the reduction in health care expenditures, the ACA contains numerous provisions to be implemented through 2018. There can be no assurance at this time that the implementation of these provisions will not have a material adverse effect on our business.
The ACA provided for states to create health insurance “Marketplaces” where individuals can compare and enroll in Qualified Health Plans, or QHPs. Individuals with an income less than 400% of the federal poverty level that purchase insurance on a Marketplace may be eligible for federal subsidies to cover a portion of their health insurance premium costs and cost sharing of co‑insurance or co‑pay obligations. Our patients may be enrolled in QHPs, and we may begin to submit bills to QHPs for services we provide. The presence of federal funds in QHPs in
25
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
the form of subsidies and cost-sharing may subject providers to heightened government attention and enforcement, which could significantly increase the cost of compliance and could materially impact our operations. For example, it is not clear whether the availability of these federal subsidies classifies a QHP as a federal healthcare program, particularly for purposes of federal fraud and abuse laws. In letters published on October 30, 2013 and February 6, 2014, the former Secretary of the Department of Health & Human Services, or DHHS, Kathleen Sebelius, indicated that DHHS does not consider QHPs to be federal healthcare programs. However, a judge may not agree with this statement by Secretary Sebelius, and other government regulators, including, but not limited to the current of future Secretary of the DHHS, may take a different position. For example, subsequent letters from U.S. Senator Charles Grassley to Secretary Sebelius and Attorney General Eric Holder on November 7, 2013 and February 12, 2014 indicate that this issue remains an outstanding question. If QHPs are classified as federal healthcare programs, it could significantly increase our costs of compliance.
In January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, or the Budget Resolution, that authorizes the implementation of legislation that would repeal portions of the ACA. Further, in January 2017, President Trump signed an Executive Order directing federal agencies with authorities and responsibilities under the ACA to waive, defer, grant exemptions from, or delay the implementation of any provision of the ACA that would impose a fiscal or regulatory burden on states, individuals, healthcare providers, health insurers, or manufacturers of pharmaceuticals or medical devices. In December of 2017, President Trump signed into law Public Law No. 115-97, which made changes to the tax code and included, among other things, a repeal of the ACA’s penalties for the individual mandate, a provision that required individuals to buy health insurance or pay a fine. Congress also could consider subsequent legislation to replace elements of the ACA that are repealed. Additionally, the ACA continues to be challenged in a variety of lawsuits. Because of the continued uncertainty about the implementation of the ACA, there can be no assurance at this time that the implementation (or repeal) of these provisions will not have a material adverse effect on our business.
Steps taken by government payers, such as Medicare and Medicaid to control the utilization and reimbursement of healthcare services, including esoteric testing may diminish our net revenue.
We face efforts by government payers to reduce utilization as well as reimbursement for laboratory testing services. Changes in governmental reimbursement may result from statutory and regulatory changes, prospective and/or retroactive rate adjustments, administrative rulings and other policy changes.
From time to time, legislative freezes and updates affect some of our tests that are reimbursed by the Medicare program under the Medicare Physician Fee Schedule, or MPFS, or Clinical Laboratory Fee Schedule, or CLFS. The MPFS is updated on an annual basis. In the past, the MPFS was updated using a prescribed statutory formula; when application of the statutory formula resulted in lower payments, Congress has passed interim legislation to prevent the reductions. The Medicare Access and CHIP Reauthorization Act of 2015, or MACRA, repealed the previous statutory update formula and specified the update adjustment factors for calendar years 2015 and beyond. If the updated conversion factor results in negative reimbursement in future years, the resulting decrease in payment may adversely affect our revenue, business, operating results, financial condition and prospects.
In addition, recent laws have made changes to Medicare reimbursement for our tests that are reimbursed under the CLFS, many of which have already gone into effect. In June 2016, CMS published the Clinical Laboratory Fee CLFS final rule entitled “Medicare Program: Medicare Clinical Diagnostic Laboratory Tests Payment System” (CMS-1621-F). The final rule provides regulations to implement the provisions of the Protecting Access to Medicare Act of 2014, or PAMA, which was signed to law in April 2014. Under the final rule, laboratories, including physician office laboratories, are required to report private payer rate and volume data if they:
•Have more than $12,500 in Medicare revenues from laboratory services on the CLFS and
•They receive more than 50 percent of their Medicare revenues from laboratory and physician services during a data collection period.
Tests that meet the criteria for being considered new advanced tests will be paid at actual list charge during an initial period of three calendar quarters. Once the initial period is over, payment for new, advanced tests would be based on
26
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
the weighted median private payer rate reported by the single laboratory that performs the new ADLT. Advanced tests are tests furnished by only one laboratory that include a unique algorithm and, at a minimum, are an analysis of RNA, DNA or proteins or are cleared or approved by the FDA.
Applicable laboratories must report data that includes the payment rate (reflecting all discounts, rebates, coupons and other price concessions) and the volume of each test that was paid by each private payer (including health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations). The definition of “applicable” lab may exclude certain types of laboratories that generally received more favorable pricing than other laboratories, and thus the make-up of laboratories reporting pricing data to CMS under the proposed rule may result in lower overall pricing data. Beginning in 2017, the Medicare payment rate for each clinical diagnostic lab test is equal to the weighted median amount for the test from the most recent data collection period. For example, laboratories were required to collect private payer data from January 1, 2016 through June 30, 2016 and report it to CMS by March 31, 2017. The new Medicare CLFS rates (based on weighted median private payer rates) was released in November 2017 and were effective on January 1, 2018. Also for the years 2017 through 2019, the amount of reduction in the Medicare rate (if any) shall not exceed 10 percent from the prior year’s rate and for the years 2020 through 2022, any reduction shall not exceed 15 percent from the prior year’s rate. It is too early to predict the impact on reimbursement for our tests reimbursed under the CLFS, though we believe the government’s goal is to reduce Medicare program payments for CLFS tests. Specifically, CMS states that it anticipates the effect of the proposed rule on the Medicare program to save $360 million in program payments for CLFS tests furnished in FY 2017, and to save $5.14 billion over 10 years. CMS has also proposed that a laboratory’s failure to comply with reporting obligations, or a laboratory that makes a misrepresentation or omission in reporting required information, would be a violation of the Civil Monetary Penalties Law.
Also under PAMA, CMS is required to adopt temporary billing codes to identify new tests and new advanced diagnostic laboratory tests that have been cleared or approved by the FDA. For an existing test that is cleared or approved by the FDA and for which Medicare payment is made, CMS is required to assign a unique billing code if one has not already been assigned by the agency. Further, PAMA provides special payment status to “advanced diagnostic laboratory tests,” or ADLTs, to allow such ADLTs to be paid using their actual list charge amount during a certain time frame. We cannot determine at this time the full impact of the new law on our business, financial condition and results of operations.
CMS also adopts regulations and policies, from time to time, revising, limiting or excluding coverage or reimbursement for certain of the tests that we perform. Likewise, many state governments are under budget pressures and are also considering reductions to their Medicaid fees. Further, Medicare, Medicaid and other third party payers audit for overutilization of billed services. Even though all tests performed by us are ordered by our clients, who are responsible for establishing the medical necessity for the tests ordered, we may be subject to recoupment of payments, as the recipient of the payments for such tests, in the event that a third party payer such as CMS determines that the tests failed to meet all applicable criteria for payment. When third party payers like CMS revise their coverage regulations or policies, our costs generally increase due to the complexity of complying with additional administrative requirements. Furthermore, Medicaid reimbursement and regulations vary by state. Accordingly, we are subject to varying administrative and billing regulations, which also increase the complexity of servicing such programs and our administrative costs. Finally, state budget pressures have encouraged states to consider several courses that may impact our business, such as delaying payments, restricting coverage eligibility, service coverage restrictions and imposing taxes on our services.
In certain jurisdictions including Arkansas, Arizona, California, Hawaii, Indiana, Idaho, Iowa, Kansas, Kentucky, Michigan, Missouri, Montana, Nebraska, Nevada, North Carolina, North Dakota, Ohio, Oregon, South Carolina, South Dakota, Utah, Virginia, Washington, West Virginia and Wyoming, Medicare administrative contractors CGS Administrators, Noridian Healthcare Solutions and Palmetto GBA, administer the Molecular Diagnostic Services Program, or MolDX, and establish coverage and reimbursement for certain molecular diagnostic tests, including many of our tests. To obtain Medicare coverage for a molecular diagnostic test (FDA approved or LDT), laboratories must apply for and obtain a unique test identifier or what is known as a “Z” code. For newly developed tests or for established tests that have not been validated for clinical and analytical validity and clinical utility, laboratories must submit a detailed dossier of clinical data to substantiate that the test meets Medicare’s requirements for coverage. We have received favorable coverage for many of our molecular tests, however we have
27
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
also received non-coverage determinations for many newer tests. The field of molecular diagnostics is evolving very rapidly, and clinical studies on many new tests are still underway. We cannot be assured that some of our molecular tests will ever be covered services by Medicare, nor can we determine when the medical literature will meet the standard for coverage that Medicare administrative contractors have set.
In recent years, Medicare has encouraged beneficiaries to participate in managed care programs, known as “Medicare Advantage” programs, and has encouraged beneficiaries from the traditional fee-for- service Medicare program to switch to Medicare Advantage programs. This has resulted in rapid growth of health insurance and managed care plans offering Medicare Advantage programs and growth in Medicare beneficiary enrollment in these programs. Also in recent years, many states have increasingly mandated that Medicaid beneficiaries enroll in managed care arrangements. If these efforts continue to be successful, we may experience a further shift of traditional Medicare and Medicaid fee-for-service beneficiaries to managed care programs. As a result, we would be required to contract with those private managed care programs in order to be reimbursed for services provided to their Medicare and Medicaid members. There can be no assurance that we will be successful in entering into agreements with these managed care programs at rates of payment similar to those we realize from our non-managed care lines of business.
On January 1, 2018 CMS made changes to what is known as the “14-day rule” regarding Molecular testing. Prior to 2018, CMS’ 14-day rule prevented reference and independent laboratories such as ours from billing Medicare directly for molecular pathology tests ordered less than 14days following an outpatients discharge from the hospital. Instead, we would seek reimbursement from the hospital and the hospital would bill Medicare. Certain Molecular tests that previously were not allowed to be billed to Medicare, are now once again allowed to be billed by laboratories directly to the Medicare program. In 2017, these tests related to patients that had testing within 14 days of a hospital stay were charged directly to the referring hospital. Since our client-bill pricing is typically higher for Molecular testing than the Medicare fee schedule, we anticipate a reduction in revenue from this policy change. Under the MolDX program there are many policies that limit reimbursement on certain tests based on diagnosis codes, and for certain tests there is no reimbursement regardless of the patient’s condition.
We expect the initiatives described above to continue and, if they do, to reduce reimbursements for clinical laboratory services, to impose more stringent cost controls on clinical laboratory services and to reduce utilization of clinical laboratory services. These efforts, including changes in law or regulations that may occur in the future, may each individually or collectively have a material adverse impact on our business, results of operations, financial condition and prospects.
Changes in regulations, payer policies or contracting arrangements with payers or changes in other laws, regulations or policies may adversely affect coverage or reimbursement for our specialized diagnostic services, which may decrease our revenues and adversely affect our results of operations and financial condition.
Governmental payers, as well as private insurers and private payers, have implemented and will continue to implement measures to control the cost, utilization and delivery of healthcare services, including clinical laboratory and pathology services. Congress and federal agencies, such as CMS, have, from time to time, implemented changes to laws and regulations governing healthcare service providers, including specialized diagnostic service providers. These changes have adversely affected and may in the future adversely affect coverage for our services. We also believe that healthcare professionals may not use our services if third-party payers do not provide adequate coverage and reimbursement for them. These changes in federal, state, local and third-party payer regulations or policies may decrease our revenues and adversely affect our results of operations and financial condition. We will continue to be a non-contracting provider until such time as we enter into contracts with third-party payers with whom we are not currently contracted. Because a portion of our revenues is from third-party payers with whom we are not currently contracted, it is likely that we will be required to make positive or negative adjustments to accounting estimates with respect to contractual allowances in the future, which may adversely affect our results of operations, our credibility with financial analysts and investors, and our stock price.
28
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
Clinical trials and research services create a risk of liability.
Errors or omissions could occur during a clinical trial that may result in harm to study volunteers, or if unnoticed and regulatory approval received, to consumers of the drug, or that undermine the usefulness of the clinical trial or data from the clinical trial and may delay the entry of a drug to the market.
Our contracts include provisions entitling us to be indemnified or entitling us to a limitation of liability. These provisions do not uniformly protect us against liability arising from certain of our own actions, such as gross negligence or misconduct. We could be materially and adversely affected if we were required to pay damages or bear the costs of defending any claim which is not covered by a contractual indemnification provision or in the event that a party who must indemnify us does not fulfill its indemnification obligations or which is beyond the level of our insurance coverage. There can be no assurance that we will be able to maintain such insurance coverage on terms acceptable to us.
We may not be able to implement our business strategy, which could impair our ability to continue operations.
Implementation of our business strategies will depend in large part on our ability to (i) attract and maintain a significant number of clients; (ii) effectively provide acceptable products and services to our clients; (iii) develop and license new products and technologies; (iv) obtain adequate financing on favorable terms to fund our business strategies; (v) maintain appropriate internal procedures, policies, and systems; (vi) hire, train, and retain skilled employees and management; (vii) continue to operate despite increasing competition in the medical laboratory industry; (viii) be paid reasonable fees by government payer’s that will adequately cover our costs; (ix) establish, develop and maintain our name recognition; and (x) establish and maintain beneficial relationships with third-party insurance providers and other third-party payers. Our inability to obtain or maintain any or all these factors could impair our ability to implement our business strategies successfully, which could have material adverse effects on our results of operations and financial condition.
We may be unsuccessful in managing our growth which could prevent us from operating profitably.
Our growth has placed, and is expected to continue to place, a significant strain on our managerial, operational and financial resources. To manage our expanded business and our potential growth, we must continue to implement and improve our operational, financial and billing systems and to expand, train and manage our employee base. We may not be able to effectively manage the expansion of our operations and our systems, procedures or controls may not be adequate to support our operations. Our management may not be able to achieve the rapid execution necessary to fully exploit the market opportunity for our products and services. Any inability to manage growth could have a material adverse effect on our business, results of operations, potential profitability and financial condition.
We have a substantial amount of indebtedness. This level of indebtedness could adversely affect our flexibility in operating our business and our ability to react to changes in the economy or our industry.
In December 2016, we entered into a senior secured revolving credit facility, providing for up to $150 million of borrowings, comprised of a $75 million senior secured term loan facility and a $75 million revolving loan. At December 31, 2017, we had $96.7 million of indebtedness outstanding, and approximately $16.7 million of available borrowing capacity under our senior secured revolving credit facility. The revolving credit facility allows for additional borrowings as long as the debt to Adjusted EBITDA ratio remains below 3.75 for 2017, 3.50 for 2018, and as specified in the respective agreements for future years. The full amount of borrowings under the term loan facility and $22.9 million of borrowings under the revolving credit facility were used to retire the then existing term loan and redeem $55 million in shares of our convertible and redeemable (“Series A Preferred Stock”) received by an affiliate of General Electric (GE Medical) in connection with our acquisition of Clarient (“the Acquisition”). Our substantial indebtedness could have significant consequences for our business and financial condition. For example:
29
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
|
|
investments in new technologies, make stock repurchases and fund other general corporate purposes. If we fail to meet our payment obligations or otherwise fail to comply with the covenants in our debt, including failure as a result of events beyond our control, it could result in an event of default on our debt. Upon an event of default, the lenders of that debt could elect to cause all amounts outstanding with respect to that debt to become immediately due and payable and we would be unable to access our revolving credit facility. Our debt imposes operating and financial covenants and restrictions on us, and compliance with such covenants and restrictions may adversely affect our ability to adequately finance our operations or capital needs, pursue attractive business opportunities that may arise, redeem or repurchase capital stock, pay dividends, sell assets, and make capital expenditures. |
|
|
• |
We may experience increased vulnerability to general adverse economic conditions, including increases in interest rates for those borrowings that bear interest at variable rates or if such indebtedness is refinanced at a time when interest are higher. |
|
|
• |
We may experience limited flexibility in planning for, or reacting to, changes in or challenges relating to our businesses and industry, creating competitive disadvantages compared to other competitors with lower debt levels and borrowing costs. |
We cannot assure you that cash flows, combined with additional borrowings under the revolving credit facility or any future credit facility, will be available in an amount sufficient to enable us to repay our indebtedness, or to fund other liquidity needs.
In addition, we may incur substantial additional indebtedness in the future, which could cause the related risks to intensify. We may need to refinance all or a portion of our indebtedness on or before their respective maturities. We cannot assure you that we will be able to refinance any of our indebtedness on commercially reasonable terms or at all. If we are unable to refinance our debt, we may default under the terms of our indebtedness, which could lead to an acceleration of the debt. We do not expect that we could repay all of our outstanding indebtedness if the repayment of such indebtedness was accelerated.
In addition, for so long as any shares of our Series A Preferred Stock remain outstanding, in the event that we issue any other shares of capital stock or any unsecured debt securities for cash, we are required to apply at least 50% of the net cash proceeds to redeem shares of Series A Preferred Stock at the then-effective liquidation preference, which is $7.50 per share as of the date of this report, less any applicable redemption discounts. As a result, our ability to repay our outstanding indebtedness will be constrained by the fact that we will only receive half of the net cash proceeds from certain capital raising activities for as long as any shares of our Series A Preferred Stock remains outstanding.
If we are unable to successfully integrate any future business we may acquire, with our legacy business, the anticipated benefits of such transaction may not be realized.
Acquisitions, involve the combination of two companies that formerly operated as independent companies. Acquisitions require us to devote significant management attention and resources to integrating the acquired company’s business practices and operations with our own. Potential difficulties we may encounter as part of the integration process, all of which could materially and adversely affect our business, financial condition, results of operations, and cash flows, include the following:
|
|
• |
the potential inability to successfully combine the acquired company’s business with our legacy business in a manner that permits us to achieve the cost synergies expected to be achieved when expected, or at all, and other benefits anticipated to result from such transaction; |
|
|
• |
challenges optimizing the customer information and technology of the two companies, including the goal of consolidating to one laboratory information system and one billing system; |
|
|
• |
challenges effectuating any diversification strategy, including challenges achieving revenue growth from sales of each company’s products and services to the customers of the other company; |
|
|
• |
difficulties offering products and services across our expanded portfolio; |
30
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
|
|
• |
the need to revisit assumptions about reserves, revenues, capital expenditures, and operating costs, including expected synergies; |
|
|
• |
challenges faced by a potential diversion of the attention of our management as a result of the integration, which in turn could adversely affect our ability to maintain relationships with customers, employees and other constituencies or our ability to achieve the anticipated benefits of such transaction; |
|
|
• |
the potential loss of key employees, customers, managed care contracts or strategic partners, or the ability to attract or retain key management and other key personnel, which could have an adverse effect on our ability to integrate and operate the acquired business; |
|
|
• |
complexities associated with managing the combined businesses, including difficulty addressing possible differences in corporate cultures and management philosophies and the challenge of integrating complex systems, technology, networks and other assets of each of the companies in a seamless manner that minimizes any adverse impact on customers, suppliers, employees and other constituencies; |
|
|
• |
costs and challenges related to the integration of the acquired company’s internal controls over financial reporting with ours; and |
|
|
• |
potential unknown liabilities and unforeseen increased expenses. |
We cannot be assured that all of the goals and anticipated benefits of an acquisition, will be achievable, particularly as the achievement of the benefits are in many important respects subject to factors that we do not control. These factors would include such things as the reactions of third parties with whom we enter into contracts and to business and the reactions of investors and analysts.
If we cannot integrate our business and any future business we may acquire, successfully, we may fail to realize the expected benefits of such transaction, including the anticipated cost synergies. We could also encounter additional transaction and integration costs or be subject to other factors that affect preliminary estimates.
Other manufacturers may discontinue or recall testing products used in our business.
We rely heavily on reagents, test kits and instruments manufactured by third parties in our testing services. From time to time, manufacturers discontinue or recall the reagents, test kits or instruments used by us to perform laboratory testing. Such discontinuations or recalls could adversely affect our costs, testing volume, costs and revenues.
Failure to develop, or acquire licenses for, new or improved testing technologies could materially and adversely affect our revenues.
Our industry is subject to changing technology and new product introductions. Other companies or individuals, including our competitors, may obtain patents or other property rights that would prevent, limit or interfere with our ability to develop, perform or sell our solutions or operate our business or increase our costs. In addition, they could introduce new tests, technologies or services that may result in a decrease in the demand for our services or cause us to reduce the prices of our services. Our success will depend, in part, on our ability to develop, acquire or license new and improved technologies on favorable terms and to obtain appropriate coverage and reimbursement for these technologies. We may not be able to negotiate acceptable licensing arrangements and we cannot be certain that such arrangements will yield commercially successful diagnostic tests. If we are unable to license these testing methods at competitive rates, our research and development costs may increase as a result. In addition, if we are unable to license new or improved technologies to expand our testing operations, our testing methods may become outdated when compared with our competition and testing volume and revenue may be materially and adversely affected.
31
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
We may incur greater costs than anticipated, which could result in sustained losses.
We use reasonable efforts to assess and predict the expenses necessary to pursue our business strategies. However, implementing our business strategies may require more employees, capital equipment, supplies or other expenditure items than management has predicted, particularly as we continue to assess any further needs resulting from the Acquisition. Similarly, the cost of compensating additional management, employees and consultants or other operating costs may be more than we estimate, which could result in ongoing and sustained losses.
We may face fluctuations in our results of operations and we are subject to seasonality in our business which could negatively affect our business operations.
Management expects that our results of operations may fluctuate significantly in the future as a result of a variety of factors, including, but not limited to: (i) the continued rate of growth, usage and acceptance of our products and services; (ii) demand for our products and services; (iii) the introduction and acceptance of new or enhanced products or services by us or by competitors; (iv) our ability to anticipate and effectively adapt to developing markets and to rapidly changing technologies; (v) our ability to attract, retain and motivate qualified personnel; (vi) the initiation, renewal or expiration of significant contracts with any major clients; (vii) pricing changes by us, our suppliers or our competitors; (viii) seasonality; and (ix) general economic conditions and other factors. Accordingly, future sales and operating results are difficult to forecast. Our expenses are based in part on our expectations as to future revenues and to a significant extent are relatively fixed, at least in the short-term. We may not be able to adjust spending in a timely manner to compensate for any unexpected revenue shortfall. Accordingly, any significant shortfall in relation to our expectations would likely have an immediate adverse impact on our business, results of operations and financial condition. In addition, we may determine from time to time to make certain pricing or marketing decisions or acquisitions that could have a short-term material adverse effect on our business, results of operations and financial condition and may not result in the long-term benefits intended. Furthermore, in Florida, historically our largest referral market for lab testing services, a meaningful percentage of the population, returns to homes in the Northern United States to avoid the hot summer months. This combined with the usual summer vacation schedules of our clients usually results in seasonality in our business. Because of all of the foregoing factors, our operating results in future periods could be less than the expectations of investors.
We depend substantially upon third parties for payment of services, which could have a material adverse effect on our cash flows and results of operations.
Our business consists of clinical laboratories that provide medical testing services for doctors, hospitals, and other laboratories on patient specimens that are sent to our laboratory. In the case of some specimen referrals that are received for patients that are not in-patients or out-patients at a hospital or institution or otherwise sent by another reference laboratory, we typically bill the patient’s insurance company or a government program for our services. As such, we rely on the cooperation of numerous third-party payers, including but not limited to Medicare, Medicaid, and various insurance companies, to get paid for performing services on behalf of our clients and their patients. The amount of such third-party payments is governed by contractual relationships in cases where we are a participating provider for a specified insurance company or by established government reimbursement rates in cases where we are an approved provider for a government program such as Medicare or Medicaid. However, we do not have contractual relationships with some of the insurance companies with whom we deal, nor are we necessarily able to become an approved provider for all government programs. In such cases, we are deemed to be a non-participating provider and there is no contractual assurance that we will be able to collect the amounts billed to such insurance companies or government programs. Currently, we are not a participating provider with some of the insurance companies we bill for our services. Until such time we become a participating provider with such insurance companies, there can be no contractual assurance that we will be paid for the services we bill to such insurance companies or patients, and such third-parties may change their reimbursement policies for non-participating providers in a manner that may have a material adverse effect on our cash flow or results of operations. When new Current Procedural Terminology (“CPT”) codes are introduced by the American Medical Association it often takes time for commercial insurance providers to recognize the new codes, which can significantly impact the timing of payments, if any, and can increase our days-sales-outstanding. Medicare has also, at times, issued codes or coding guidance that conflicts with the AMA CPT coding, which can cause confusion when secondary insurance is
32
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
involved. Insurance companies may also try to steer business away from us towards in-network providers by sending letters to physicians and even imposing financial penalties if they continue to send us business.
The market for our services is highly competitive, which could have a material adverse effect on our business, results of operations and financial condition.
The market for genetic and molecular testing services is highly competitive and we expect competition to continue to increase. We compete with other commercial clinical laboratories in addition to the in-house laboratories of many major hospitals and physician practices. Many of our existing competitors have significantly greater financial, human, technical and marketing resources than we do. Some physician groups and hospitals have made the decision to internalize testing rather than using an outsourced laboratory such as us and therefore control the referral of their own specimens. Our competitors may develop products and services that are superior to ours or that achieve greater market acceptance than our offerings. We may not be able to compete successfully against current and future sources of competition and in such cases, this may have a material adverse effect on our business, results of operations and financial condition.
Increased competition, including price competition, could have a material adverse impact on our net revenues and profitability.
Our industry is characterized by intense competition. Our major competitors including Quest Diagnostics and Laboratory Corporation of America are large national laboratories that possess greater name recognition, larger customer bases, and significantly greater financial resources and employ substantially more personnel than we do. Many of our competitors have long established relationships with their customers and third-party payers. We cannot assure you that we will be able to compete successfully with such entities in the future.
The laboratory business is intensely competitive both in terms of price and service. Pricing of laboratory testing services is often one of the most significant factors used by health care providers and third-party payers in selecting a laboratory. As a result of the laboratory industry undergoing consolidation, larger laboratory providers are able to increase cost efficiencies afforded by large-scale automated testing. This consolidation results in greater price competition. We may be unable to increase cost efficiencies sufficiently, if at all, and as a result, our net earnings and cash flows could be negatively impacted by such price competition. Additionally, we may also face changes in fee schedules, competitive bidding for laboratory services or other actions or pressures reducing payment schedules as a result of increased or additional competition.
Additional competition, including price competition, could have a material adverse impact on our net revenues and profitability.
We face the risk of capacity constraints, which could have a material adverse effect on our business, results of operations and financial condition.
We compete in the market place primarily on three factors: i) the quality and accuracy of our test results; ii) the speed or turn-around times of our testing services; and iii) our ability to provide after-test support to those physicians requesting consultation. Any unforeseen increase in the volume of clients could strain the capacity of our personnel and systems, leading to unacceptable turn-around times, or customer service failures. In addition, as the number of our clients and specimens increases, our products, services, and infrastructure may not be able to scale accordingly. We may also not be able to hire additional licensed medical technologists that we need to handle increased volumes. Any failure to handle higher volume of requests for our products and services could lead to the loss of established clients and have a material adverse effect on our business, results of operations and financial condition. If we produce inaccurate test results, our clients may choose not to use us in the future. This could severely harm our business, results of operations and financial condition. In addition, based on the importance of the subject matter of our tests, inaccurate results could result in improper treatment of patients, and potential liability for us.
33
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
We may fail to protect our facilities, which could have a material adverse effect on our business, results of operations and financial condition.
Our operations are dependent in part upon our ability to protect our laboratory operations against physical damage from explosions, fire, floods, hurricanes, earthquakes, power loss, telecommunications failures, break-ins and similar events. We do not presently have an emergency back-up generator in place at our Tampa, Florida, Nashville, Tennessee, or Fresno California laboratories locations that would otherwise mitigate to some extent the effects of a prolonged power outage. The occurrence of any of these events could result in interruptions, delays or cessations in service to clients, which could have a material adverse effect on our business, results of operations and financial condition.
The steps we have taken to protect our proprietary rights may not be adequate, which could result in infringement or misappropriation by third-parties.
We regard our copyrights, trademarks, trade secrets and similar intellectual property as critical to our success, and we rely upon trademark and copyright law, trade secret protection and confidentiality and/or license agreements with our employees, clients, partners and others to protect our proprietary rights. The steps taken by us to protect our proprietary rights may not be adequate or third parties may infringe or misappropriate our copyrights, trademarks, trade secrets and similar proprietary rights. In addition, other parties may assert infringement claims against us.
We are dependent on key personnel and need to hire additional qualified personnel in order for our business to succeed.
Our performance is substantially dependent on the performance of our senior management and key technical personnel. In particular, our success depends substantially on the continued efforts of our senior management team, which currently is composed of a small number of individuals. The loss of the services of any of our executive officers, our medical staff, our laboratory directors or other key employees could have a material adverse effect on our business, results of operations and our financial condition. Our future success also depends on our continuing ability to attract and retain highly qualified managerial and technical personnel as we grow. Competition for such personnel is intense and we may not be able to retain our key managerial and technical employees or may not be able to attract and retain additional highly qualified managerial and technical personnel in the future. The inability to attract and retain the necessary managerial and technical personnel could have a material adverse effect upon our business, results of operations and financial condition.
The failure to obtain necessary additional capital to finance growth and capital requirements, could adversely affect our business, financial condition and results of operations.
We may seek to exploit business opportunities that require more capital than we have currently available. We may not be able to raise such capital on favorable terms or at all, and may be restricted in amount and type of such capital by the agreements governing our existing indebtedness. If we are unable to obtain such additional capital, we may be required to reduce the scope of our anticipated expansion, which could adversely affect our business, financial condition and results of operations.
As of December 31, 2017, we had cash and cash equivalents of approximately $12.8 million and approximately $16.7 million in available borrowing capacity under our senior secured revolving credit facility. We may still need additional capital to fully implement our business, operating and development plans. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, there could be a material adverse effect on our long-term business, rate of growth, operating results, financial condition and prospects.
34
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
If we were required to conduct additional clinical trials prior to continuing to sell our current tests or launching any other tests we may develop, those trials could result in delays or failure to obtain necessary regulatory approvals, which could harm our business.
In the event that, in the future, the FDA begins to regulate our tests, it may require additional pre- market clinical testing prior to submitting a regulatory notification or application for commercial sales. Such pre-market clinical testing could delay the commencement or completion of clinical testing, significantly increase our test development costs, delay commercialization of any future tests, and interrupt sales of our current tests. Many of the factors that may cause or lead to a delay in the commencement or completion of clinical trials may also ultimately lead to delay or denial of regulatory clearance or approval. The commencement of clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites and the eligibility criteria for the clinical trial.
We may find it necessary to engage contract research organizations to perform data collection and analysis and other aspects of our clinical trials, which might increase the cost and complexity of our trials. We may also depend on clinical investigators, medical institutions and contract research organizations to perform the trials. If these parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, or if the quality, completeness or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or for other reasons, our clinical trials may have to be extended, delayed or terminated. Many of these factors would be beyond our control. We may not be able to enter into replacement arrangements without undue delays or considerable expenditures. If there are delays in testing or approvals as a result of the failure to perform by third parties, our research and development costs would increase, and we may not be able to obtain regulatory clearance or approval for our tests. In addition, we may not be able to establish or maintain relationships with these parties on favorable terms, if at all. Each of these outcomes would harm our ability to market our tests and/or to achieve sustained profitability.
Failure in our information technology systems could significantly increase testing turn-around time or billing processes and otherwise disrupt our operations.
Our laboratory operations depend, in part, on the continued performance of our information technology systems. Our information technology systems are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptions. Sustained system failures or interruption of our systems in one or more of our laboratory operations could disrupt our ability to process laboratory requisitions, perform testing, provide test results in a timely manner and/or bill the appropriate party. Breaches with respect to protected health information could result in violations of the Health Insurance Portability and Accountability Act of 1996, or HIPAA, the Health Information Technology for Economic and Clinical Health Act, or the HITECH Act, and analogous state laws, and risk the imposition of significant fines and penalties. Failure of our information technology systems could adversely affect our business, results of operations and financial condition.
Failure to comply with environmental, health and safety laws and regulations, including the federal Occupational Safety and Health Administration Act, and the Needlestick Safety and Prevention Act could result in fines and penalties and loss of licensure, and have a material adverse effect upon our business.
We are subject to licensing and regulation under federal, state and local laws and regulations relating to the protection of the environment and human health and safety, including laws and regulations relating to the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials, as well as regulations relating to the safety and health of laboratory employees. The federal Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for health care employers, including clinical laboratories, whose workers may be exposed to blood-borne pathogens such as HIV and the hepatitis B virus. These requirements, among other things, require work practice controls, protective clothing and equipment, training, medical follow-up, vaccinations and other measures designed to minimize exposure to, and transmission of, blood-borne pathogens. In addition, the Needlestick Safety and Prevention Act requires, among other things, that we include in our safety programs the evaluation and use of engineering controls such as safety needles, if found to be effective at reducing the risk of needlestick injuries in the workplace.
35
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
Failure to comply with such federal, state and local laws and regulations could subject us to denial of the right to conduct business, fines, criminal penalties and/or other enforcement actions, any of which could have a material adverse effect on our business. In addition, compliance with future legislation could impose additional requirements for us, which may be costly.
Our net revenue will be diminished if payers do not adequately cover or reimburse our services.
There has been and will continue to be significant efforts by both federal and state agencies to reduce costs in government healthcare programs and otherwise implement government control of healthcare costs. In addition, increasing emphasis on managed care in the United States may continue to put pressure on the pricing of healthcare services. Uncertainty exists as to the coverage and reimbursement status of new applications or services. Third party payers, including governmental payers such as Medicare and private payers, are scrutinizing new medical products and services and may not cover or may limit coverage and the level of reimbursement for our services. Third party insurance coverage may not be available to patients for any of our existing tests or for tests we discover and develop. In addition, a substantial portion of the testing for which we bill our hospital and laboratory clients is ultimately paid by third party payers. Any pricing pressure exerted by these third party payers on our clients may, in turn, be exerted by our clients on us. If government and other third party payers do not provide adequate coverage and reimbursement for our tests, it could adversely affect our operating results, cash flows or and/or financial condition.
Third party billing is extremely complicated and results in significant additional costs to us.
Billing for laboratory services is extremely complicated. The customer refers the tests; the payer pays for the tests, and the two may not be the same. Depending on the billing arrangement and applicable laws, we must bill various payers, such as patients, insurance companies, Medicare, Medicaid, doctors and employer groups, hospitals and other laboratories, all of which have different billing requirements. Additionally, we undertake internal audits to evaluate compliance with applicable laws and regulations as well as internal compliance policies and procedures. Insurance companies and government payers such as Medicare and Medicaid also impose routine external audits to evaluate payments, which adds further complexity to the billing process.
Among others, the primary factors which complicate our billing practices are:
|
|
• |
pricing differences between our fee schedules and the reimbursement rates of the payers; |
|
|
• |
changes in payer rules; |
|
|
• |
disputes with payers as to the party who is responsible for payment; |
|
|
• |
disparity in coverage and information requirements among various carriers; and |
|
|
• |
differing pre-authorization requirements across insurance carriers |
We incur significant additional costs as a result of our participation in the Medicare and Medicaid programs, as billing and reimbursement for clinical laboratory services are subject to considerable and complex federal and state regulations. The additional costs we expect to incur include those related to: (i) complexity added to our billing processes and systems; (ii) training and education of our employees and clients; (iii) implementing compliance procedures and oversight; (iv) collections and legal costs; and (v) costs associated with, among other factors, challenging coverage and payment denials and providing patients with information regarding claims processing and services, such as advance beneficiary notices.
Our operations are subject to strict laws prohibiting fraudulent billing and other abuse, and our failure to comply with such laws could result in substantial penalties.
Of particular importance to our operations are federal and state laws prohibiting fraudulent billing and providing for the recovery of overpayments. In particular, if we fail to comply with federal and state documentation, coding and billing rules, we could be subject to liability under the federal False Claims Act, including criminal and/or civil penalties, loss of licenses and exclusion from the Medicare and Medicaid programs. The False Claims Act prohibits
36
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
individuals and companies from knowingly submitting false claims for payments to, or improperly retaining overpayments from, the government.
If an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 and $11,000 for each separate false claim. Further, False Claims Act liability may lead to exclusion from participation in Medicare, Medicaid and other federal healthcare programs. There are a number of potential bases for liability under the federal False Claims Act. For example, liability arises when an entity knowingly submits, or causes another to submit, a claim for reimbursement to the federal government for a service which was not provided or which did not qualify for reimbursement. Submitting a claim with reckless disregard or deliberate ignorance of its truth or falsity could also result in liability under the False Claims Act. The False Claims Act’s “whistleblower” or “qui tam” provisions are being used with more frequency to challenge the reimbursement practices of providers and suppliers. Those provisions allow a private individual to bring an action on behalf of the government alleging that the defendant has submitted false claims for payment to the federal government. The government must decide whether to intervene in the lawsuit and whether to prosecute the case. If it declines to do so, the individual may pursue the case alone, although the government must be kept apprised of the progress of the lawsuit. Whether or not the federal government intervenes in the case, it will receive the majority of any recovery. The successful qui tam relator who brought the case is entitled to a portion of the proceeds and its attorneys’ fees and costs. In addition, various states have enacted laws modeled after the federal False Claims Act, which prohibit submitting false claims for payment to the state or, in some states, to other commercial payers.
Government investigations of clinical laboratories have been ongoing for a number of years and are expected to continue in the future. When we submit bills for our services to third‑party payers, we must follow complex documentation, coding and billing rules which are based on federal and state laws, rules and regulations, various government publications, and on industry practice. A large number of laboratories have entered into substantial settlements with the federal and state governments for alleged noncompliance under these laws and rules. Private payers have also brought civil actions against laboratories which have resulted in substantial judgments. Failure to follow these rules could result in potential civil liability under the False Claims Act, under which extensive financial penalties can be imposed. It could further result in criminal liability under various federal and state criminal statutes. For example, there are various state and federal laws and rules regulating laboratory billing practices, such as prohibiting a clinical laboratory from charging a higher price for tests ordered by a physician and provided by a third party (anti-markup rules) as well as requiring direct billing of certain laboratory services by the laboratory performing the tests instead of allowing the laboratory to bill the ordering clinician for the test (direct billing rules).
We submit thousands of claims for Medicare and other payments and we cannot guarantee that there have not been errors in our claims, While we maintain a robust compliance program that includes consistent, detailed review of our documentation, coding and billing practices, the rules are frequently vague, complex, and continually changing and we cannot assure that governmental investigators, private insurers or private whistleblowers will not challenge our practices. Such a challenge could result in a material adverse effect on our business.
The failure to comply with significant government regulation and laboratory operations may subject us to liability, penalties or limitation of operations.
We are subject to extensive state and federal regulatory oversight. Specifically, our laboratories must satisfy federal requirements under the CLIA to maintain the appropriate CLIA Certificate for all testing performed at the lab. Additionally, most states have adopted various laws and regulation setting standards for laboratories performing clinical laboratory testing and requiring laboratories to obtain and maintain a state laboratory license before the laboratory is authorized to perform testing. These state licensure laws often address permissible and prohibited practices involving digital health, including but not limited to telehealth and telepathology.
37
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
Upon periodic inspection or survey, our laboratory locations may be found to be non-compliant with CLIA requirements or with applicable licensure or certification laws. The sanctions for failure to comply with CLIA, state licensure requirements, or other applicable laws and regulations could include the suspension, revocation, or limitation of the right to perform clinical laboratory services or receive compensation for those services, as well as the requirement to enter into a corrective action plan to monitor compliance, and the imposition of civil or criminal penalties or administrative fines. In addition, any new legislation or regulation or the application of existing laws and regulations in ways that we have not anticipated could have a material adverse effect on our business, results of operations and financial condition.
Existing federal laws governing Medicare and Medicaid, as well as some other state and federal laws, also regulate certain aspects of the relationship between healthcare providers, including clinical laboratories, and their referral sources, including physicians, hospitals and other laboratories. Certain of these laws, known as the federal “anti-kickback law” and the federal physician self-referral laws (also known as the “Stark Law”) contain extremely broad proscriptions. Violation of these laws may result in criminal penalties, exclusion from participation in the Medicare, Medicaid, and other federal healthcare programs, and significant civil monetary penalties, as well as False Claims Act liability. We seek to structure our arrangements with physicians and other clients to be in compliance with the anti-kickback laws, Stark Law and similar state laws, and to keep up-to-date on developments concerning their application by various means, including consultation with legal counsel and review of the annual Work Plan by the Office of the Inspector General (“OIG”) identifying targeted issues. We cannot guarantee, however, that government authorities will not take a contrary view and impose civil monetary penalties and exclude us based on our arrangements with physicians and other clients.
The federal Civil Monetary Penalties Law, or the federal CMP Law, imposes civil monetary penalties and exclusion from Medicare and Medicaid programs on any person who offers or transfers remuneration to any patient who is a Medicare or Medicaid beneficiary, when the person knows or should know that the remuneration is likely to induce the patient to receive medical services from a particular provider. The federal CMP Law applies, among other things, to many kinds of inducements or benefits provided to patients, including complimentary items, services or transportation that are of more than a nominal value. We have structured our operations and provision of services to patients in a manner that we believe complies with the law and its interpretation by government authorities. We cannot guarantee, however, that government authorities will not take a contrary view and impose civil monetary penalties and exclude us for past or present practices.
Furthermore, HIPAA, the HITECH Act, and associated regulations and similar state laws contain provisions that require the electronic exchange of health information, such as claims submission and receipt of remittances, using standard transactions and code sets, which we refer to as Standards, and regulate the use and disclosure of patient records and other Protected Health Information, or PHI. These provisions, which address security and confidentiality of patient information as well as the administrative aspects of claims handling, have very broad applicability and they specifically apply to many healthcare providers, including physicians and clinical laboratories. Although we believe we are in material compliance with the Standards, Security and Privacy rules under HIPAA and the HITECH Act and state privacy and security laws, a failure to comply with these laws could have a material adverse effect on our business, results of operations and financial condition and subject us to liability. Additionally, the amendments to HIPAA in the HITECH Act provide that the state Attorneys General may bring an action against a covered entity, such as us, for a violation of HIPAA.
The failure to comply with physician self‑referral laws may subject us to liability, penalties or limitation of operations
We are subject to the federal Stark Law, as well as similar state statutes and regulations, which prohibit payments for certain health care services, which are referred to as designated health services or (“DHS”), rendered as a result of referrals by physicians to DHS entities with which the physicians (or immediate family members) have a financial relationship. A “financial relationship” includes both an ownership interest and/or a compensation arrangement with a physician, both direct and indirect, and DHS includes, but is not limited to, laboratory services. The Stark Law prohibits an entity that receives a prohibited DHS referral from seeking payment from Medicare for any DHS services performed as a result of such a referral, unless an arrangement is carefully structure to satisfy every requirement of a regulatory exception. The Stark Law is a strict liability statute, and thus any technical violation
38
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
requires repayment of all “tainted” referrals, regardless of the intent. Penalties for violating the Stark Law may include the denial of payment to an entity for the impermissible provision of DHS, the requirement to refund any amounts collected in violation of the Stark Law, and civil monetary penalties of up to $15,000 for each violation and $100,000 for each circumvention arrangement or scheme. Other implications of a Stark Law violation may include criminal penalties, exclusion from Medicare and Medicaid programs, and potential False Claims Act liability, including via “qui tam” action.
Further, many states have promulgated self‑referral laws and regulations similar to the federal Stark Law, but these vary significantly based on the state. In addition to services reimbursed by Medicaid or government payers, often these state laws and regulations can encompass services reimbursed by private payers as well. Penalties for violating state self-referral laws and regulations vary based on the state, but often include civil and criminal penalties, exclusion from Medicaid, and loss of licenses.
Our financial arrangements with physicians are governed by the federal Stark Law, and we rely on certain exceptions to the Stark Law with respect to such relationships. While we believe that our financial relationships with physicians and referral practices are in compliance with applicable laws and regulations, we cannot guarantee that government authorities would agree. If we are found by the government to be in violation of the Stark Law, we could be subject to significant penalties, including fines as specified above, exclusion from participation in government and private payer programs and requirements to refund amounts previously received from government. Further, as our operations expand into new states and jurisdictions, we must continually evaluate whether our relationships with physicians comply with that jurisdiction’s laws. This may require structural and organizational modifications to our relationships with physicians which could adversely affect our results of operations and financial condition.
The failure to comply with Anti-Kickback laws may subject us to liability, penalties or limitation of operations
We are subject to the federal Anti-Kickback Statute, or the AKS, as well as similar state statutes and regulations, which prohibit the offer, payment, solicitation or receipt of any form of remuneration in return for referring, ordering, leasing, purchasing or arranging for or recommending the ordering, purchasing or leasing of items or services payable by Medicare, Medicaid or any other federally funded healthcare program. The AKS defines remuneration to include anything of value, in cash or in kind, and thus can implicate financial relationships including payments not commensurate with fair market value, such as in the form of space, equipment leases, professional or technical services or anything else of value.
The AKS is an “intent‑based” statute, meaning that a violation occurs when one or both parties intend the remuneration to be in exchange for or to induce referrals; however, the ACA, among other things, amended the intent requirement of the AKS. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the ACA provides that the government may assert that a claim including items or services resulting from a violation of the AKS constitutes a false or fraudulent claim for purposes of the false claims statutes. There are a number of statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions; however, the exceptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exception or safe harbor may be subject to scrutiny. Violations of the AKS may result in substantial civil or criminal penalties, including criminal fines of up to $25,000, imprisonment of up to five years, civil penalties under the federal CMP Law of up to $50,000 for each violation, plus three times the remuneration involved, civil penalties under the federal False Claims Act of up to $11,000 for each claim submitted, plus three times the amounts paid for such claims and exclusion from participation in the Medicare and Medicaid programs. If we face these penalties or the participation exclusion, it could significantly reduce our revenues and could have a material adverse effect on our business.
Further, most states have adopted similar anti-kickback laws prohibiting the offer, payment, solicitation or receipt of remuneration in exchange for referrals, and typically impose criminal and civil penalties as well as loss of licenses. Some of these state laws apply to items and services paid for by private payers as well as to government payers. In addition, many states have adopted laws prohibiting the splitting or sharing of fees between physicians and non‑physicians, as well as between treating physicians and referral sources. We believe our arrangements with
39
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
physicians comply with the AKS, and state anti-kickback and fee‑splitting laws of the states in which we operate, however, if government regulatory authorities were to disagree, we could be subject to civil and criminal penalties, and be required to restructure or terminate our contractual and other arrangements with physicians. This could result in a loss of revenue and have a material adverse effect on our business.
Some states have also adopted laws prohibiting the corporate practice of medicine, or prohibiting business corporations from employing physicians or engaging in activities considered to be the “practice of medicine.” In these states, we rely on service agreements with physicians and/or professional associations owned by physicians, to perform needed professional pathology services. We cannot assure you that a physician or physician’s professional organization will not seek to terminate an agreement with us on any basis, nor can we assure you that governmental authorities in those states will not seek termination of these arrangements on the basis of state laws prohibiting the corporate practice of medicine.
A failure to comply with governmental payer regulations could result in our being excluded from participation in Medicare, Medicaid or other governmental payer programs.
Tests which are reimbursed by Medicare and other Government payers (for example, State Medicaid programs) accounted for approximately 15%, 16% and 21% of our revenues for the years ended December 31, 2017, 2016 and 2015, respectively. The Medicare program imposes extensive and detailed requirements on diagnostic service providers, including, but not limited to, rules that govern how we structure our relationships with physicians, how and when we submit claims for reimbursement and how we provide specialized diagnostic laboratory services. Further, we are prohibited from contracting with any individuals or entities who have been excluded from participation in Medicare or Medicaid and are listed on the OIG’s List of Excluded Individuals and Entities List. Contracting with excluded individuals or entities, such as hiring an excluded person or contracting with an excluded vendor, can result in significant penalties.
Our failure to comply with applicable Medicare, Medicaid and other governmental payer rules could result in our inability to participate in a governmental payer program, an obligation to repay funds already paid to us for services performed, civil monetary penalties, criminal penalties, False Claims Act liability and/or limitations on the operational function of our laboratory. If we were unable to receive reimbursement under a governmental payer program, a substantial portion of our revenues would be lost, which would adversely affect our results of operations and financial condition.
Failure to comply with the HIPAA Privacy, Security and Breach Notification Regulations may increase our operational costs.
The HIPAA privacy and security regulations establish comprehensive federal standards with respect to the uses and disclosures of PHI by certain entities including health plans and health care providers, and set standards to protect the confidentiality, integrity and availability of electronic PHI. The regulations establish a complex regulatory framework on a variety of subjects, including, for example, the circumstances under which uses and disclosures of PHI are permitted or required without a specific authorization by the patient; a patient’s right to access, amend and receive an accounting of certain disclosures of PHI; the content of notices of privacy practices describing how PHI is used and disclosed and individuals’ rights with respect to their PHI; and implementation of administrative, technical and physical safeguards to protect privacy and security of PHI. Recent revisions to HIPAA allow patients the option to obtain certain of their test reports directly from the laboratory, instead of learning the results from the ordering physician. We have implemented policies and procedures to comply with the HIPAA privacy and security laws and regulations. The privacy regulations establish a uniform federal standard but do not supersede state laws that may be more stringent. Therefore, we are required to comply with both federal privacy and security regulations and varying state privacy and security laws and regulations. The federal privacy regulations restrict our ability to use or disclose certain individually identifiable patient health information, without patient authorization, for purposes other than payment, treatment or health care operations (as defined by HIPAA), except for disclosures for various public policy purposes and other permitted purposes outlined in the privacy regulations.
The HITECH Act and its implementing regulations also require healthcare providers like us to notify affected individuals, the Secretary of the U.S. Department of Health and Human Services, and in some cases, the media,
40
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
when PHI has been breached as defined under and following the requirements of HIPAA. Many states have similar breach notification laws. In the event of a breach, we could incur operational and financial costs related to remediation as well as preparation and delivery of the notices, which costs could be substantial. Additionally, HIPAA, the HITECH Act, and their implementing regulations provide for significant civil fines, criminal penalties, and other sanctions for failure to comply with the privacy, security, and breach notification rules, including for wrongful or impermissible use or disclosure of PHI. Although the HIPAA statute and regulations do not expressly provide for a private right of action for damages, we could incur damages under state laws to private parties for the wrongful or impermissible use or disclosure of confidential health information or other private personal information. Additionally, amendments to HIPAA provide that the state Attorneys General may bring an action against a covered entity, such as us, for a violation of HIPAA. We insure some of our risk with respect to HIPAA security breaches although there could be operational costs associated with HIPAA breaches above our insured limits.
We are subject to security risks which could harm our operations.
HIPAA and the HITECH Act imposed additional requirements, restrictions and penalties on covered entities and their business associates to, among other things, deter breaches of security. As a result, the remedial actions required, the reporting requirements, and sanctions for a breach are stringent. Our electronic health records system is periodically modified to meet applicable security standards. Despite the implementation of various security measures by us, our infrastructure may be vulnerable to computer viruses, break-ins and similar disruptive problems caused by our clients or others, which could lead to interruption, delays or cessation in service to our clients. Further, such incidents, whether electronic or physical could also potentially jeopardize the security of confidential information, including PHI stored in our computer systems as it relates to clients, patients, and other parties connected through us, which may deter potential clients and give rise to uncertain liability to parties whose security or privacy has been infringed. A significant security breach could result in fines, loss of clients, damage to our reputation, direct damages, costs of repair and detection, costs to remedy the breach, and other expenses. We insure some of our risk with respect to security breaches but the occurrence of any of the foregoing events could have a material adverse effect on our business, results of operations and financial condition.
Clinicians or patients using our services may sue us, and our insurance may not sufficiently cover all claims brought against us, which will increase our expenses.
The development, marketing, sale and performance of healthcare services expose us to the risk of litigation, including professional negligence or product liability claims were someone to allege that our tests failed to perform as designed. We may also be subject to liability for errors in the test results we provide to pathologists and oncologists or for a misunderstanding of, or inappropriate reliance upon, the information we provide. Damages assessed in connection with, and the costs of defending, any legal action could be substantial. We may be faced with litigation claims that exceed our insurance coverage or are not covered under any of our insurance policies. In addition, litigation could have a material adverse effect on our business if it impacts our existing and potential customer relationships, creates adverse public relations, diverts management resources from the operation of the business, or hampers our ability to otherwise conduct our business.
We must hire and retain qualified sales representatives to grow our sales, if not, our existing business and our results of operations and financial condition will likely suffer.
Our ability to retain existing clients for our specialized diagnostic services and attract new clients is dependent upon retaining existing sales representatives and hiring and training new sales representatives, which is an expensive and time-consuming process. We face intense competition for qualified sales personnel and our inability to hire or retain an adequate number of sales representatives could limit our ability to maintain or expand our business and increase sales. Even if we are able to increase our sales force, our new sales personnel may not commit the necessary resources or provide sufficient high quality service and attention to effectively market and sell our services. If we are unable to maintain and expand our marketing and sales networks or if our sales personnel do not perform to our standards, we may be unable to maintain or grow our existing business and our results of operations and financial condition will likely suffer accordingly. If a sales representative ceases employment, we risk the loss of client goodwill based on the impairment of relationships developed between the sales representative and the healthcare professionals for whom the sales representative was responsible. This is particularly a risk if the representative goes
41
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
to work for a competitor, as the healthcare professionals that are our clients may choose to use a competitor’s services based on their relationship with our former sales representative.
Further, non-compliant activities and unlawful conduct by sales and marketing personnel could give rise to significant risks under the AKS. We require extensive, comprehensive training of all sales and marketing personnel, but cannot guarantee that every staff member will comply with the training. Thus, in addition to the cost of training sales and marketing personnel, we could face liability under the AKS for non-compliance by individuals engaged in prohibited sales and marketing activities.
Performance issues, service interruptions or price increases by our shipping carrier could adversely affect our business, results of operations and financial condition, and harm our reputation and ability to provide our specialized diagnostic services on a timely basis
Expedited, reliable shipping is essential to our operations. One of our marketing strategies entails highlighting the reliability of our point-to-point transport of patient samples. We rely heavily on a single provider of transport services, FedEx Corporation, or the Carrier, for reliable and secure point-to-point transport of patient samples to our laboratory and enhanced tracking of these patient samples. Should the Carrier encounter delivery performance issues such as loss, damage or destruction of a sample, it may be difficult to replace our patient samples in a timely manner and such occurrences may damage our reputation and lead to decreased demand for our services and increased cost and expense to our business. In addition, any significant increase in shipping rates could adversely affect our operating margins and results of operations. Similarly, strikes, severe weather, natural disasters or other service interruptions by delivery services we use would adversely affect our ability to receive and process patient samples on a timely basis. If the Carrier or we were to terminate our relationship, we would be required to find another party to provide expedited, reliable point-to-point transport of our patient samples. There are only a few other providers of such nationwide transport services, and there can be no assurance that we will be able to enter into arrangements with such other providers on acceptable terms, if at all. Finding a new provider of transport services would be time-consuming and costly and result in delays in our ability to provide our specialized diagnostic services. Even if we were to enter into an arrangement with such provider, there can be no assurance that they will provide the same level of quality in transport services currently provided to us by the Carrier. If the new provider does not provide the required quality and reliable transport services, it could adversely affect our business, reputation, results of operations and financial condition.
We use biological and hazardous materials that require considerable expertise and expense for handling, storage or disposal and may result in claims against us
We work with hazardous materials, including chemicals, biological agents and compounds, blood samples and other human tissue that could be dangerous to human health and safety or the environment. Our operations also produce hazardous and bio hazardous waste products. Federal, state and local laws and regulations govern the use, generation, manufacture, storage, handling and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair business efforts. If we do not comply with applicable regulations, we may be subject to fines and penalties. In addition, we cannot entirely eliminate the risk of accidental injury or contamination from these materials or wastes. Our general liability insurance and/or workers’ compensation insurance policy may not cover damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could be held liable for damages or penalized with fines in an amount exceeding our resources, and our operations could be suspended or otherwise adversely affected.
Risks Relating to Our Common Stock
Future sales of our common stock by GE Medical, or the perception that such sales may occur, could cause our stock price to decline.
The shares of common stock we issued or which we may issue upon conversion of Series A Preferred Stock to GE Medical as consideration in the Acquisition are restricted, but GE Medical may sell such shares under certain circumstances. Under the Investor Board Rights, Lockup and Standstill Agreement, GE Medical’s ability to sell its
42
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
shares of our common stock is limited for the specified lockup period, subject to volume limitations under Rule 144 under the Securities Act of 1933 and other exceptions. Furthermore, under the Registration Rights Agreement with GE Medical we are required to file, upon expiration of a lockup period, a registration statement for the resale of common stock by GE Medical, which registration statement when declared effective will allow GE Medical to sell a significant number of shares of our common stock in a short period of time. The sale of a substantial number of shares of our common stock by GE Medical or our other stockholders or the perception that such sales may occur could cause our stock price to decline, make it more difficult for us to raise funds through future offerings of our common stock or acquire other businesses using our common stock as consideration.
As a result of the Acquisition, GE Medical has significant influence over us and actions requiring general stockholder approval.
As a result of the Acquisition, GE Medical owns approximately 19% of our total voting power based on the number of shares of common stock outstanding as of March 5, 2018. This percentage may increase upon the conversion of shares of Series A Preferred Stock (including any additional shares of Series A Preferred Stock issued as payment-in-kind dividends into common stock) if such preferred stock is not first redeemed. In connection with the Acquisition, GE Medical Systems has the right to designate one individual for approval and we are required to appoint such designee, as a director to our Board of Directors. In addition, the Investor Board Rights, Lockup And Standstill Agreement with GE Medical contains certain rights in favor of GE Medical, including requiring GE Medical’s approval before we can further increase the size of our Board of Directors and providing GE Medical with the right to participate in future rights offerings to our current stockholders as if the Series A Preferred Stock issued to GE Medical had been converted into shares of common stock. The terms of the Series A Preferred Stock issued to GE Medical provide that, without GE Medical’s consent, we may not, among other things, repurchase outstanding shares of our common stock, or engage in certain other transactions.
As a result, GE Medical will have significant influence over matters requiring stockholder approval, including future amendments to our Amended and Restated Articles of Incorporation or other significant or extraordinary transactions. GE Medical’s interests may differ from the interests of our other shareholders with respect to certain matters.
In addition, having GE Medical as a significant stockholder may make it more difficult for a third party to acquire, or discourage a third party from seeking to acquire, a majority of our outstanding shares of common stock or control of the Board of Directors through a proxy solicitation.
We currently do not expect to pay any cash dividends and the price of our stock may not appreciate.
We do not anticipate paying dividends on our common stock in the foreseeable future. Rather, we plan to retain earnings, if any, for the operation and expansion of our business. If we do not pay dividends, the price of our common stock must appreciate for you to recognize a gain on your investment upon sale. This appreciation may not occur.
We may become involved in securities class action litigation that could divert management’s attention and harm our business.
The stock markets have from time to time experienced significant price and volume fluctuations that have affected the market prices for the common stock of diagnostic companies. These broad market fluctuations may cause the market price of our common stock to decline. In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because clinical laboratory service companies have experienced significant stock price volatility in recent years. We may become involved in this type of litigation in the future. Litigation often is expensive and diverts management’s attention and resources, which could adversely affect our business.
43
NEOGENOMICS, INC.
ITEM 1A. RISK FACTORS (CONTINUED)
If any securities analyst downgrades our common stock or our sector, the price of our common stock could be negatively affected.
Securities analysts may publish reports about us or our industry containing information about us that may affect the trading price of our common stock. If a securities or industry analyst downgrades the outlook for our common stock or one of our competitors’ stocks or chooses to terminate coverage of our common stock, the trading price of our common stock may be negatively affected.
The price of our common stock may fluctuate significantly.
The price of our common stock has been, and is likely to continue to be, volatile, which means that it could decline substantially within a short period of time. The price of our common stock could fluctuate significantly for many reasons including the following:
|
|
• |
future announcements concerning us or our competitors; |
|
|
• |
regulatory developments and enforcement actions bearing on advertising, marketing or sales; |
|
|
• |
reports and recommendations of analysts and whether or not we meet the milestones and metrics set forth in such reports; gaining or losing large customers or managed care plans; |
|
|
• |
introduction of new products or services and related insurance coverage; |
|
|
• |
acquisition or loss of significant manufacturers, distributors or suppliers or an inability to obtain sufficient quantities of materials needed to provide our services; |
|
|
• |
quarterly variations in operating results; |
|
|
• |
business acquisitions or divestitures; |
|
|
• |
changes in the regulation of Laboratory Developed Tests (“LDTs”); |
|
|
• |
changes in governmental or third-party reimbursement practices and rates; and fluctuations in the economy, political events or general market conditions. |
In addition, stock markets in general and the market for shares of health care stocks in particular, have experienced extreme price and volume fluctuations in recent years, fluctuations that frequently have been unrelated to the operating performance of the affected companies. These broad market fluctuations may adversely affect the market price of our common stock. The market price of our common stock could decline below its current price and the market price of our shares may fluctuate significantly in the future. These fluctuations may be unrelated to our performance.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None
44
NEOGENOMICS, INC.
We operate a regional network of laboratories. Our corporate office and all our laboratory facilities are leased; these leases expire at various dates through 2022. We believe that these locations are sufficient to meet our needs at existing volume levels and that, if needed, additional space will be available at a reasonable cost. The following table summarizes our facilities by type and location:
|
Location |
|
Purpose |
|
Square Footage |
|
|
|
Aliso Viejo, California |
|
Laboratory, and administrative offices |
|
|
96,917 |
|
|
Fort Myers, Florida |
|
Corporate headquarters and laboratory |
|
|
51,729 |
|
|
Houston, Texas |
|
Laboratory |
|
|
24,330 |
|
|
Geneva-Rolle, Switzerland |
|
Laboratory |
|
|
7,976 |
|
|
Nashville, Tennessee |
|
Laboratory |
|
|
7,806 |
|
|
Tampa, Florida |
|
Laboratory |
|
|
5,875 |
|
|
Fresno, California |
|
Laboratory |
|
|
2,541 |
|
|
Atlanta, Georgia |
|
Laboratory |
|
|
1,190 |
|
|
Plantation, Florida |
|
Courier office |
|
|
240 |
|
Our Rolle, Switzerland laboratory supports our Pharma Services segment exclusively; all other locations support both segments of our business. We anticipate moving into our new facility currently under construction in Houston, Texas in the second quarter of 2018. This new facility will have 28,143 square feet and we will vacate our current Houston facility. For further financial information about our segments, see Note Q to our Consolidated Financial Statements included in this Annual Report.
From time to time the Company is engaged in legal proceedings in the ordinary course of business. We do not believe any current legal proceedings are material to our business. No material proceedings were terminated in the fourth quarter of 2017.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
45
NEOGENOMICS, INC.
ITEM 5. MARKET FOR THE REGISTRANTS COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is listed on the NASDAQ Capital Market under the symbol “NEO”. Set forth below is a table summarizing the high and low sales price per share for our common stock during the periods indicated.
|
|
|
High Sales Price |
|
|
Low Sales Price |
|
||
|
2017 |
|
|
|
|
|
|
|
|
|
4th Quarter 2017 |
|
$ |
11.47 |
|
|
$ |
7.82 |
|
|
3rd Quarter 2017 |
|
11.63 |
|
|
8.62 |
|
||
|
2nd Quarter 2017 |
|
|
9.22 |
|
|
7.12 |
|
|
|
1st Quarter 2017 |
|
|
9.06 |
|
|
|
7.50 |
|
|
2016 |
|
|
|
|
|
|
|
|
|
4th Quarter 2016 |
|
$ |
9.88 |
|
|
$ |
6.90 |
|
|
3rd Quarter 2016 |
|
|
9.54 |
|
|
|
7.79 |
|
|
2nd Quarter 2016 |
|
|
9.17 |
|
|
|
6.56 |
|
|
1st Quarter 2016 |
|
|
8.00 |
|
|
|
5.49 |
|
The above table is based on information provided by NASDAQ Capital Market. These quotations reflect inter-dealer prices, without retail mark-up, markdown or commissions, and may not necessarily represent actual transactions. All historical data was obtained from the www.nasdaq.com web site.
Holders of Common Stock
As of March 5, 2018, there were 497 stockholders of record of our common stock. The number of record holders does not include beneficial owners of common stock whose shares are held in the names of banks, brokers, nominees or other fiduciaries.
Dividends
We have never declared or paid cash dividends on our common stock. We intend to retain all future earnings to finance operations and future growth and, therefore, we do not anticipate paying any cash dividends in the foreseeable future. Our financing arrangements contain certain restrictions on our ability to pay dividends on our common stock. In addition, the Certificate of Designations governing the Series A Preferred Stock that we issued in December 2015 restricts us from declaring and paying certain dividends on our common stock without the prior written consent of Holders of a majority of the shares of Series A Preferred Stock. In addition, Holders of Series A Convertible Preferred Stock shall be entitled to a proportionate share of any distributions as though they were the holders of the number of shares of common stock into which their shares convert into.
46
NEOGENOMICS, INC.
ITEM 5. MARKET FOR THE REGISTRANTS COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES (CONTINUED)
Equity Compensation Plan Information
The following table summarizes the securities authorized for issuance under equity compensation plans as of December 31, 2017:
|
Plan Category |
|
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
|
|
Weighted average exercise price of outstanding options, warrants and rights |
|
|
Number of securities remaining available for future issuance under equity compensation plans |
|
|
|||
|
Equity compensation plans approved by security holders: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amended and Restated Equity Incentive Plan (“Equity Incentive Plan”) |
|
|
6,342,526 |
|
|
$ |
6.51 |
|
|
|
5,440,222 |
|
(a) |
|
Employee Stock Purchase Plan (“ESPP”) |
|
|
— |
|
|
N/A |
|
|
|
132,566 |
|
|
|
|
Equity compensation plans not approved by security holders |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
Total |
|
|
6,342,526 |
|
|
$ |
6.51 |
|
|
|
5,572,788 |
|
|
|
(a) |
The Company’s Equity Incentive Plan was amended, restated and subsequently approved by a majority of shareholders on April 16, 2013, May 4, 2015, December 21, 2015 and most recently on May 25, 2017. The most recent amendment increased the maximum aggregate number of shares of the Company’s common stock reserved and available for issuance under the Amended Plan to 18,650,000. |
Currently, the Company’s Equity Incentive Plan, as amended and restated on May 25, 2017 and the Company’s ESPP, as Amended and Restated on May 25, 2017, are the only equity compensation plans in effect.
Recent Sales of Unregistered Securities
On December 30, 2015 we issued 15,000,000 shares of common stock and 14,666,667 shares of Series A Convertible Preferred Stock to GE Medical in connection with the acquisition of Clarient, Inc., and we entered into a registration rights agreement in order to establish certain rights and restrictions related to the registration of the shares. See Notes D and H to our financial statements. There were no unregistered sales of equity in 2016 or 2017.
47
NEOGENOMICS, INC.
ITEM 5. MARKET FOR THE REGISTRANTS COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES (CONTINUED)
Comparison of Cumulative Five Year Total Return
We have presented below the cumulative total return to our stockholders of $100 during the period from December 31, 2012, through December 31, 2017 in comparison to the cumulative return on the S&P 500 Index and a customized peer group of 7 publicly traded companies during that same period. The peer group is made up of Cancer Genetics, Inc., Enzo Biochem, Inc., Genomic Health, Inc., Foundation Medicine, Laboratory Corporation of America Holdings, Myriad Genetics, Inc., and Quest Diagnostics, Inc. Several of our closest competitors are part of large pharmaceutical or other multi-national firms, or are privately held and, as such, we are unable to get financial information for them.
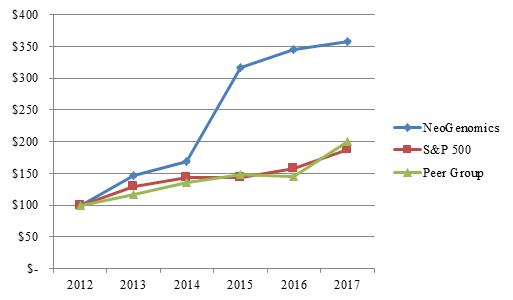
The results assume that $100 (with reinvestment of all dividends) was invested in our common stock, the index and in the peer group and its relative performance tracked through December 31, 2017. The comparisons are based on historical data and are not indicative of, nor intended to forecast, the future performance of our common stock. The performance graph set forth above shall not be deemed incorporated by reference into any filing by us under the Securities Act or the Exchange Act except to the extent that we specifically incorporate such information by reference therein.
48
NEOGENOMICS, INC.
ITEM 6. SELECTED FINANCIAL DATA
The following is a summary of our historical consolidated financial data for the periods ended and at the dates indicated below. You are encouraged to read this information together with our audited consolidated financial statements and the related footnotes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this Annual Report.
The historical consolidated financial data for the years ended December 31, 2017, 2016 and 2015 (Statement of Operations Data and Other Cash Data) has been derived from our audited consolidated financial statements, which are included elsewhere in this Annual Report. The historical consolidated financial data for the years ended December 31, 2014 and 2013 has been derived from our audited consolidated financial statements, which are not included in this Annual Report.
The historical consolidated financial data as of December 31, 2017 and 2016 (Balance Sheet Data) has been derived from our audited consolidated financial statements, which are included elsewhere in this Annual Report. The historical consolidated financial data (Balance Sheet Data) as of December 31, 2015, 2014 and 2013 has been derived from our audited consolidated financial statements, which are not included in this Annual Report.
We believe that the comparability of our financial results between the periods presented in the table below is significantly impacted by factors which are more fully described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements and the notes thereto included elsewhere in this Annual Report.
|
|
|
Years Ended December 31, |
|
|||||||||||||||||
|
|
|
2017 (3) |
|
|
2016 |
|
|
2015 (1) |
|
|
2014 (2) |
|
|
2013 |
|
|||||
|
|
|
(In thousands, except per share data) |
|
|||||||||||||||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net revenue |
|
$ |
258,611 |
|
|
$ |
244,083 |
|
|
$ |
99,802 |
|
|
$ |
87,069 |
|
|
$ |
66,467 |
|
|
Cost of revenue |
|
|
138,295 |
|
|
|
133,704 |
|
|
|
56,046 |
|
|
|
46,355 |
|
|
|
34,730 |
|
|
Gross margin |
|
|
120,316 |
|
|
|
110,379 |
|
|
|
43,756 |
|
|
|
40,714 |
|
|
|
31,737 |
|
|
Operating expenses |
|
|
116,934 |
|
|
|
107,805 |
|
|
|
49,391 |
|
|
|
38,496 |
|
|
|
28,563 |
|
|
Income (loss) from operations |
|
|
3,382 |
|
|
|
2,574 |
|
|
|
(5,635 |
) |
|
|
2,218 |
|
|
|
3,174 |
|
|
Interest and other income (expense) |
|
|
(6,863 |
) |
|
|
(9,998 |
) |
|
|
1,146 |
|
|
|
(929 |
) |
|
|
(989 |
) |
|
Income tax (benefit) expense |
|
|
(2,635 |
) |
|
|
(1,701 |
) |
|
|
(1,954 |
) |
|
|
157 |
|
|
|
152 |
|
|
Net income (loss) |
|
|
(846 |
) |
|
|
(5,723 |
) |
|
|
(2,535 |
) |
|
|
1,132 |
|
|
|
2,033 |
|
|
Deemed dividends on preferred stock |
|
|
3,645 |
|
|
|
18,011 |
|
|
|
40 |
|
|
|
- |
|
|
|
- |
|
|
Amortization of preferred stock beneficial conversion feature |
|
|
6,902 |
|
|
|
6,663 |
|
|
|
82 |
|
|
|
- |
|
|
|
- |
|
|
Net income (loss) due to common stockholders |
|
$ |
(11,393 |
) |
|
$ |
(30,397 |
) |
|
$ |
(2,657 |
) |
|
$ |
1,132 |
|
|
$ |
2,033 |
|
|
Net income (loss) per common share – Basic |
|
$ |
(0.14 |
) |
|
$ |
(0.39 |
) |
|
$ |
(0.04 |
) |
|
$ |
0.02 |
|
|
$ |
0.04 |
|
|
Net income (loss) per common share – Diluted |
|
$ |
(0.14 |
) |
|
$ |
(0.39 |
) |
|
$ |
(0.04 |
) |
|
$ |
0.02 |
|
|
$ |
0.04 |
|
|
Other Cash Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash – operating activities |
|
$ |
18,037 |
|
|
$ |
21,477 |
|
|
$ |
6,393 |
|
|
$ |
9,450 |
|
|
$ |
2,227 |
|
|
Net cash – investing activities |
|
$ |
(13,690 |
) |
|
$ |
(6,501 |
) |
|
$ |
(75,155 |
) |
|
$ |
(9,602 |
) |
|
$ |
(2,011 |
) |
|
Net cash – financing activities |
|
$ |
(4,095 |
) |
|
$ |
(25,871 |
) |
|
$ |
58,493 |
|
|
$ |
29,007 |
|
|
$ |
2,750 |
|
|
(1) |
Reflects the acquisition of Clarient in December 2015. |
|
(2) |
Reflects the acquisition of Path Logic in July 2014. |
|
(3) |
Reflects the sale of Path Logic on August 1, 2017. |
49
NEOGENOMICS, INC.
ITEM 6. SELECTED FINANCIAL DATA (CONTINUED)
|
|
|
As of December 31, |
|
|||||||||||||||||
|
|
|
2017 (4) |
|
|
2016 |
|
|
2015 (1)(3) |
|
|
2014 (2) |
|
|
2013 |
|
|||||
|
|
|
(In thousands) |
|
|||||||||||||||||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets |
|
$ |
84,963 |
|
|
$ |
78,825 |
|
|
$ |
82,360 |
|
|
$ |
58,742 |
|
|
$ |
27,491 |
|
|
Property and equipment |
|
|
36,504 |
|
|
|
34,036 |
|
|
|
34,577 |
|
|
|
15,082 |
|
|
|
9,694 |
|
|
Intangible assets |
|
|
74,165 |
|
|
|
77,064 |
|
|
|
87,800 |
|
|
|
4,212 |
|
|
|
2,577 |
|
|
Goodwill |
|
|
147,019 |
|
|
|
147,019 |
|
|
|
146,421 |
|
|
|
2,929 |
|
|
|
— |
|
|
Other assets |
|
|
689 |
|
|
|
174 |
|
|
|
129 |
|
|
|
141 |
|
|
|
154 |
|
|
Total assets |
|
$ |
343,340 |
|
|
$ |
337,118 |
|
|
$ |
351,287 |
|
|
$ |
81,106 |
|
|
$ |
39,916 |
|
|
Current liabilities |
|
$ |
35,065 |
|
|
$ |
38,113 |
|
|
$ |
40,058 |
|
|
$ |
14,623 |
|
|
$ |
14,323 |
|
|
Long-term liabilities |
|
|
102,742 |
|
|
|
112,409 |
|
|
|
73,117 |
|
|
|
6,078 |
|
|
|
3,882 |
|
|
Total liabilities |
|
|
137,807 |
|
|
|
150,522 |
|
|
|
113,175 |
|
|
|
20,701 |
|
|
|
18,205 |
|
|
Series A Redeemable Convertible Preferred Stock |
|
|
32,615 |
|
|
|
22,873 |
|
|
|
28,602 |
|
|
|
— |
|
|
|
— |
|
|
Stockholders’ equity |
|
|
172,918 |
|
|
|
163,723 |
|
|
|
209,510 |
|
|
|
60,405 |
|
|
|
21,711 |
|
|
Total liabilities preferred stock and stockholders’ equity |
|
$ |
343,340 |
|
|
$ |
337,118 |
|
|
$ |
351,287 |
|
|
$ |
81,106 |
|
|
$ |
39,916 |
|
|
Working Capital |
|
$ |
49,898 |
|
|
$ |
40,712 |
|
|
$ |
42,302 |
|
|
$ |
44,119 |
|
|
$ |
13,168 |
|
|
(1) |
Reflects the acquisition of Clarient in December 2015. |
|
(2) |
Reflects the acquisition of Path Logic in July 2014. |
|
(3) |
Reflects the adoption of ASU 2015-17. |
|
(4) |
Reflects the sale of Path Logic on August 1, 2017. |
50
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with the Consolidated Financial Statements, and the Notes thereto included in this Annual Report on Form 10-K. The information contained below includes statements of management’s beliefs, expectations, hopes, goals and plans that, if not historical, are forward-looking statements subject to certain risks and uncertainties that could cause actual results to differ materially from those anticipated in the forward-looking statements. For a discussion on forward-looking statements, see the information set forth in the Introductory Note to this Annual Report under the caption “Forward Looking Statements”, which information is incorporated herein by reference.
Our Company
NeoGenomics, Inc. is a high-complexity CLIA-certified clinical laboratory that specializes in cancer genetics diagnostic testing. The Company's testing services include cytogenetics, fluorescence in-situ hybridization (FISH), flow cytometry, immunohistochemistry, anatomic pathology and molecular genetic testing. Headquartered in Fort Myers, FL, NeoGenomics has laboratories in Aliso Viejo and Fresno, CA; Tampa and Fort Myers, FL; Houston, TX; Nashville, TN and Rolle, Switzerland. NeoGenomics services the needs of pathologists, oncologists, other clinicians and hospitals throughout the United States and Europe.
2017 Overview and Highlights
|
|
• |
We completed the integration of Clarient by combining facilities and systems during 2017. |
|
|
• |
We increased clinical test volume by approximately 17% in 2017 compared to 2016. |
|
|
• |
We opened our first international laboratory location in Rolle, Switzerland in November of 2017, which offered Pharma Services to international clients. |
|
|
• |
We increased Pharma revenue by approximately 22% in 2017 compared to 2016. |
|
|
• |
We reduced cost per clinical test year-over-year by approximately 11%. |
|
|
• |
We completed a full renovation of our Aliso Viejo, CA laboratory, significantly increasing our testing capacity. |
Company Outlook
We have developed a company-wide focus for 2018, which includes the following three critical success factors:
|
|
• |
To strengthen our world-class culture by improving teamwork and emphasizing effective communication. We will focus on career development and mobility through mentoring and training opportunities to enhance and capitalize on the talent within our Company. |
|
|
• |
To provide uncompromising quality through company-wide leadership, training and employee engagement. Our laboratory teams will focus on quality by improving corrective and preventative metrics in the laboratory. |
|
|
• |
To pursue exceptional service and growth through developing cross functional teams to analyze key market segments and engaging customers within these segments to determine ways to further drive growth and pursue excellent service. We will continue to pursue market share gains in both our Clinical and Pharma Services businesses. |
These critical success factors have been communicated throughout our Company. We have structured departmental goals around these factors and have created employee incentive plans in which every employee will have a meaningful incentive for our success.
51
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
As we focus on profitable growth, we will aggressively pursue large purchasing group contracts. In 2017, we were successful in gaining market share by entering into contracts with managed care organizations and large hospital groups, partially due to the benefits of scale achieved by the Clarient acquisition. This will continue to be part of our strategy going forward. In addition, our molecular testing menu remains a strong selling point as it enables us to offer clients a “one stop shop” where they can send all of their oncology testing rather than using multiple labs.
Innovation and changes in science and technology will lead to new therapeutic and diagnostic tests. Our Company will strive to lead in innovation with continued expansion of our test menu for oncology and expansion of liquid biopsy tests. We will continue to work with pharmaceutical clients on their clinical trials and will work to be on the leading edge of developments in the field of oncology.
We believe lower cost and increased value of testing is extremely important to the healthcare industry and creates a competitive advantage for our company. We will invest in information technology, automation and best practices to continually drive down the cost of testing. We will continue to expand our test menu and remain at the forefront of the ongoing revolution in cancer related genetic and molecular testing to achieve our vision of becoming the world’s leading cancer testing and information company.
We are significantly expanding our capacity, specifically in the Pharma Services area of our business. The opening of our laboratory in Rolle, Switzerland as well as the expansion of our Houston laboratory will allow us to better serve our existing Pharma Services clients and obtain new business in the U.S. and across Europe. We are also opening a small laboratory in Atlanta, Georgia, which will focus primarily on flow cytometry cases. Our strong growth momentum as well as our added capacity will create opportunities for improved quality and revenue growth.
Regulatory Environment
The FDA has been considering changes which may include increased regulation of Laboratory Developed Tests (“LDTs”). These changes could impact the laboratory testing industry and our business, as further described the discussion of Government Regulations in Item 1. In October 2014, the FDA announced its proposed framework and timetable. However, at this point the FDA has not released a proposed rule, and it is anticipated that there would be a comment period related to such a significant change. The FDA has indicated that there will be a “phase in” period that in some instances will take as long as nine years. On January 13, 2017 the FDA released a discussion paper in which the FDA said that they “hope that it advances public discussion on future LDT oversight”. The paper does not represent formal FDA policy, nor is it enforceable. Recently, Congress has submitted a legislative discussion draft, the Diagnostic Accuracy and Innovation Act (“DAIA”), to the FDA and requested technical assistance on the draft. NeoGenomics is a member of the American Clinical Laboratory Association (“ACLA”), who has been in active discussions with the FDA and Congress regarding FDA oversight of LDT’s. At this point we cannot predict the outcome of this issue, or if there will be any changes to current rules and regulations.
We closely monitor changes in legislation and take specific actions to identify and estimate the impact of changes in legislation whenever possible as regulatory changes can affect reimbursement for clinical laboratory services. We do not anticipate significant changes to our clinical revenue in 2018 based on known changes in legislation.
52
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
We analyzed our reporting structure in 2017, including the information available to our Chief Operating Decision Maker (“CODM”) and the information used to make strategic decisions. Prior to 2017, our operations were reported as one consolidated segment. Based on our 2017 analysis and due to changes made in the fourth quarter of 2017, including the opening of our first European laboratory specifically dedicated to Pharma Services clients, we changed our reporting structure to report our operations in two segments; Clinical Services and Pharma Services.
We have presented the financial information reviewed by the CODM including revenues, cost of revenue and gross margin for each of our operating segments. The segment information presented in these financial statements has been conformed to present segments on this revised basis for all prior periods. Assets are not presented at the segment level as that information is not used by the CODM.
Clinical Services
Our Clinical Services segment includes the cancer testing services we offer to community-based pathologists, hospitals, academic centers, and oncology groups and is designed to be a natural extension of, and complementary to, the services that they perform within their own practices. We believe our relationship as a non-competitive partner to community-based pathology practices, hospital pathology labs and academic centers empowers them to expand their breadth of testing and provide a menu of services that matches or exceeds the level of service found in any center of excellence around the world.
Pharma Services
Our Pharma Services segment supports pharmaceutical firms in their drug development programs by supporting various clinical trials. This portion of our business often involves working with the pharmaceutical firms (sponsors) on study design as well as performing the required testing. Our medical team often advises the sponsor and works closely with them as specimens are received from the enrolled sites. We also work on developing tests that will be used as part of a companion diagnostic to determine patients’ response to a particular drug. As studies unfold, our clinical trials team reports the data and often provide key analysis and insights back to the sponsors.
Our Pharma Services Segment provides comprehensive testing services in support of our pharmaceutical clients’ oncology programs from discovery to commercialization. In biomarker discovery, our aim is to help our customers discover the right content. We help our customers develop a biomarker hypothesis by recommending an optimal platform for molecular screening and backing our discovery tools with the informatics to capture meaningful data. In other pre and non-clinical work, we can use our platforms to characterize markers of interest. Moving from discovery to development, we help our customers refine their biomarker strategy and, if applicable, develop a companion diagnostic pathway using the optimal technology for large-scale clinical trial testing.
Whether serving as the single contract research organization or partnering with one, our Pharma group provides significant technical expertise working closely with our customers to support each stage of clinical trial development. Each trial we support comes with rapid turnaround time, dedicated project management and quality assurance oversight. We have experience in supporting submissions to the Federal Drug Administration for companion diagnostics. Our Pharma Services strategy is focused on helping bring more effective oncology treatments to market through providing world class laboratory services in oncology to key pharmaceutical companies in the industry.
Critical Accounting Policies
The preparation of financial statements in conformity with United States generally accepted accounting principles requires our management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Our management routinely makes judgments and estimates about the effects of matters that are inherently uncertain. For
53
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
a complete description of our significant accounting policies, see Note B to our Consolidated Financial Statements included in this Annual Report.
Our critical accounting policies are those where we have made difficult, subjective or complex judgments in making estimates, and/or where these estimates can significantly impact our financial results under different assumptions and conditions. Our critical accounting policies are:
|
|
• |
Revenue Recognition |
|
|
• |
Accounts Receivable and Allowance for Doubtful Accounts |
|
|
• |
Intangible Assets |
|
|
• |
Stock Based Compensation |
|
|
• |
Deferred taxes |
Revenue Recognition
The adoption of ASC 606, which is effective January 1, 2018, will require us to implement new revenue policies, procedures and internal controls related to revenue recognition and will also impact revenue in each of our segments. For further information regarding the impact to each segment, see Note B to our Consolidated Financial Statements included in this Annual Report.
For the years ended December 31, 2017, 2016 and 2015, the Company recognized revenues when (a) the price is fixed or determinable, (b) persuasive evidence of an arrangement exists, (c) the service is performed and (d) collectability of the resulting receivable is reasonably assured.
The Company’s specialized diagnostic services are performed based on a written test requisition form or electronic equivalent and revenues are recognized once the diagnostic services have been performed and the results have been delivered to the ordering physician. These diagnostic services are billed to various payers, including Medicare, commercial insurance companies, other directly billed healthcare institutions such as hospitals and clinics, and individuals. The Company reports revenues from contracted payers, including Medicare, certain insurance companies and certain healthcare institutions, based on the contractual rate, or in the case of Medicare, published fee schedules. The Company reports revenues from non-contracted payers, including certain insurance companies and individuals, based on the amount expected to be collected. The difference between the amount billed and the amount estimated to be collected from non-contracted payers is recorded as a contractual allowance to arrive at the reported net revenues. The expected revenues from non-contracted payers are based on the historical collection experience of each payer or payer group, as appropriate. The Company records revenues from patient pay tests net of a large discount and, as a result, recognizes minimal revenue on those tests. The Company regularly reviews its historical collection experience for non-contracted payers and adjusts its expected revenues for current and subsequent periods accordingly. The following table reflects our estimate of the breakdown of net revenue by type of payer for the fiscal years ended December 31, 2017, 2016, and 2015:
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Medicare and other government |
|
|
15 |
% |
|
|
16 |
% |
|
|
21 |
% |
|
Commercial insurance |
|
|
18 |
% |
|
|
25 |
% |
|
|
21 |
% |
|
Client direct billing |
|
|
64 |
% |
|
|
56 |
% |
|
|
55 |
% |
|
Patient and year-end accrual |
|
|
3 |
% |
|
|
3 |
% |
|
|
3 |
% |
|
Total |
|
|
100 |
% |
|
|
100 |
% |
|
|
100 |
% |
Our proportion of client direct billing has increased over the years shown above, as more payers, including private commercial insurances and Medicare Advantage plans are practicing “consolidated payment” or “bundled payment” models where they pay the hospitals a lump sum, which is intended to include laboratory testing. This reflects an increase in the amount of risk sharing that CMS and other private payers are encouraging providers such as hospital
54
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
systems to undertake. Our acquisition of Clarient in December of 2015 also increased our percentage of client direct billing, specifically in Pharma Services, as in that division all revenue is billed directly to clients. We had previously anticipated a gradual increase in the percentage of client direct billing over the coming years; however, on January 1, 2018 Medicare made a significant change to what is known as the “14-day rule”. The net result of this rule change is that certain molecular tests that were previously billed to clients, are now once again eligible to be billed directly to the Medicare program. We now anticipate that our Medicare direct bill revenue will increase slightly in 2018 and our client direct bill revenue will decrease slightly.
Trade Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are comprised of amounts due from sales of the Company’s specialized diagnostic services and are recorded at the invoiced amount, net of discounts and contractual allowances. The allowance for doubtful accounts is estimated based on the aging of accounts receivable with each payer category and the historical data on bad debts in these aging categories. In addition, the allowance is adjusted periodically for other relevant factors, including regularly assessing the state of our billing operations in order to identify issues which may impact the collectability of receivables or allowance estimates. Revisions to the allowance are recorded as an adjustment to bad debt expense within general and administrative expenses. After appropriate collection efforts have been exhausted, specific receivables deemed to be uncollectible are charged against the allowance in the period they are deemed uncollectible. Recoveries of receivables previously written-off are recorded as credits to the allowance.
The following tables present the Company’s gross accounts receivable by payer group at December 31, 2017 and 2016 ($ in thousands):
AGING OF RECEIVABLES BY PAYER GROUP
December 31, 2017
|
Payer Group |
|
0-30 |
|
|
% |
|
|
31-60 |
|
|
% |
|
|
61-90 |
|
|
% |
|
|
91-120 |
|
|
% |
|
|
>120 |
|
|
% |
|
|
Total |
|
|
% |
|
||||||||||||
|
Client AR - Pharma |
|
$ |
7,170 |
|
|
|
10 |
% |
|
$ |
792 |
|
|
|
1 |
% |
|
$ |
1,016 |
|
|
|
1 |
% |
|
$ |
1,030 |
|
|
|
1 |
% |
|
$ |
101 |
|
|
|
0 |
% |
|
$ |
10,109 |
|
|
|
13 |
% |
|
Client AR - Clinical |
|
|
13,624 |
|
|
|
18 |
% |
|
|
7,917 |
|
|
|
11 |
% |
|
|
4,272 |
|
|
|
6 |
% |
|
|
2,000 |
|
|
|
3 |
% |
|
|
3,411 |
|
|
|
5 |
% |
|
|
31,224 |
|
|
|
43 |
% |
|
Total Client AR |
|
$ |
20,794 |
|
|
|
|
|
|
$ |
8,709 |
|
|
|
|
|
|
$ |
5,288 |
|
|
|
|
|
|
$ |
3,030 |
|
|
|
|
|
|
$ |
3,512 |
|
|
|
|
|
|
$ |
41,333 |
|
|
|
|
|
|
Commercial insurance |
|
|
1,164 |
|
|
|
2 |
% |
|
|
1,638 |
|
|
|
2 |
% |
|
|
1,621 |
|
|
|
2 |
% |
|
|
1,339 |
|
|
|
2 |
% |
|
|
11,649 |
|
|
|
16 |
% |
|
|
17,411 |
|
|
|
24 |
% |
|
Medicaid |
|
|
145 |
|
|
|
0 |
% |
|
|
252 |
|
|
|
0 |
% |
|
|
264 |
|
|
|
0 |
% |
|
|
193 |
|
|
|
0 |
% |
|
|
956 |
|
|
|
1 |
% |
|
|
1,810 |
|
|
|
1 |
% |
|
Medicare |
|
|
1,235 |
|
|
|
2 |
% |
|
|
1,214 |
|
|
|
2 |
% |
|
|
912 |
|
|
|
1 |
% |
|
|
842 |
|
|
|
1 |
% |
|
|
5,137 |
|
|
|
7 |
% |
|
|
9,340 |
|
|
|
13 |
% |
|
Private pay |
|
|
- |
|
|
|
0 |
% |
|
|
- |
|
|
|
0 |
% |
|
|
- |
|
|
|
0 |
% |
|
|
- |
|
|
|
0 |
% |
|
|
- |
|
|
|
0 |
% |
|
|
- |
|
|
|
0 |
% |
|
Unbilled revenue |
|
|
4,047 |
|
|
|
6 |
% |
|
|
147 |
|
|
|
0 |
% |
|
|
39 |
|
|
|
0 |
% |
|
|
- |
|
|
|
0 |
% |
|
|
- |
|
|
|
0 |
% |
|
|
4,233 |
|
|
|
6 |
% |
|
Total |
|
$ |
27,385 |
|
|
|
38 |
% |
|
$ |
11,960 |
|
|
|
16 |
% |
|
$ |
8,124 |
|
|
|
10 |
% |
|
$ |
5,404 |
|
|
|
7 |
% |
|
$ |
21,254 |
|
|
|
29 |
% |
|
$ |
74,127 |
|
|
|
100 |
% |
AGING OF RECEIVABLES BY PAYER GROUP
December 31, 2016
|
Payer Group |
|
0-30 |
|
|
% |
|
|
31-60 |
|
|
% |
|
|
61-90 |
|
|
% |
|
|
91-120 |
|
|
% |
|
|
>120 |
|
|
% |
|
|
Total |
|
|
% |
|
||||||||||||
|
Client AR - Pharma |
|
$ |
2,752 |
|
|
|
4 |
% |
|
$ |
629 |
|
|
|
1 |
% |
|
$ |
305 |
|
|
|
0 |
% |
|
$ |
1,191 |
|
|
|
2 |
% |
|
$ |
421 |
|
|
|
1 |
% |
|
$ |
5,298 |
|
|
|
8 |
% |
|
Client AR - Clinical |
|
|
10,023 |
|
|
|
15 |
% |
|
|
5,891 |
|
|
|
8 |
% |
|
|
3,226 |
|
|
|
5 |
% |
|
|
1,678 |
|
|
|
2 |
% |
|
|
4,808 |
|
|
|
7 |
% |
|
|
25,626 |
|
|
|
37 |
% |
|
Total Client AR |
|
$ |
12,775 |
|
|
|
|
|
|
$ |
6,520 |
|
|
|
|
|
|
$ |
3,531 |
|
|
|
|
|
|
$ |
2,869 |
|
|
|
|
|
|
$ |
5,229 |
|
|
|
|
|
|
$ |
30,924 |
|
|
|
|
|
|
Commercial insurance |
|
|
913 |
|
|
|
1 |
% |
|
|
1,947 |
|
|
|
3 |
% |
|
|
2,045 |
|
|
|
3 |
% |
|
|
1,824 |
|
|
|
3 |
% |
|
|
11,325 |
|
|
|
16 |
% |
|
|
18,054 |
|
|
|
26 |
% |
|
Medicaid |
|
|
88 |
|
|
|
0 |
% |
|
|
203 |
|
|
|
0 |
% |
|
|
198 |
|
|
|
0 |
% |
|
|
180 |
|
|
|
0 |
% |
|
|
301 |
|
|
|
1 |
% |
|
|
970 |
|
|
|
1 |
% |
|
Medicare |
|
|
840 |
|
|
|
1 |
% |
|
|
1,300 |
|
|
|
2 |
% |
|
|
779 |
|
|
|
1 |
% |
|
|
601 |
|
|
|
1 |
% |
|
|
3,167 |
|
|
|
5 |
% |
|
|
6,687 |
|
|
|
10 |
% |
|
Private pay |
|
|
16 |
|
|
|
0 |
% |
|
|
7 |
|
|
|
0 |
% |
|
|
10 |
|
|
|
0 |
% |
|
|
10 |
|
|
|
0 |
% |
|
|
(4 |
) |
|
|
0 |
% |
|
|
39 |
|
|
|
0 |
% |
|
Unbilled revenue |
|
|
10,066 |
|
|
|
15 |
% |
|
|
1,250 |
|
|
|
2 |
% |
|
|
654 |
|
|
|
1 |
% |
|
|
225 |
|
|
|
0 |
% |
|
|
342 |
|
|
|
0 |
% |
|
|
12,537 |
|
|
|
18 |
% |
|
Total |
|
$ |
24,698 |
|
|
|
36 |
% |
|
$ |
11,227 |
|
|
|
16 |
% |
|
$ |
7,217 |
|
|
|
10 |
% |
|
$ |
5,709 |
|
|
|
8 |
% |
|
$ |
20,360 |
|
|
|
30 |
% |
|
$ |
69,211 |
|
|
|
100 |
% |
55
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
The following table represents our allowance balances at each balance sheet date presented and that allowance as a percentage of gross accounts receivable ($ in thousands):
|
|
|
December 31, |
|
|
|
|
|
|||||
|
|
|
2017 |
|
|
2016 |
|
|
$ Change |
|
|||
|
Allowance for doubtful accounts |
|
$ |
13,700 |
|
|
$ |
13,699 |
|
|
$ |
1 |
|
|
As a % of gross accounts receivable |
|
|
18.5 |
% |
|
|
19.8 |
% |
|
|
|
|
|
Days Sales Outstanding |
|
82 |
|
|
84 |
|
|
|
|
|
||
For the year ended December 31, 2017, the percentage of gross accounts receivable has decreased as compared to the year ended December 31, 2016.
Days Sales Outstanding (“DSO”) has also decreased slightly from 84 days at December 31, 2016 to 82 days at December 31, 2017. These consolidated results include a decrease in Clinical Services DSO’s from 84 days at December 31, 2016 to 79 days at December 31, 2017. This decrease is the result of improved billing operations as the Clarient integration was completed and billing operations returned to a steady state. These consolidated results also include an increase in Pharma Services DSO’s from 95 days at December 31, 2016 to 107 days at December 31, 2017. This increase is partially related to timing as many Pharma Services projects are billed upon meeting certain milestones and, therefore, the billing and collections are not consistent from month to month. In addition, there was a delay in the billing of Pharma projects in the fourth quarter, which added to the increase in Pharma Services DSO’s.
Intangible Assets
We review our long-lived assets for recoverability if events or changes in circumstances indicate the assets may be impaired. Impairment exists when the carrying amount of the asset exceeds fair value.
Clarient
As a result of the acquisition of Clarient in December 2015, see Note D to our Consolidated Financial Statements included in this Annual Report, we recorded an estimated $84.0 million in intangible assets comprised of $81.0 million in customer relationships amortized over a fifteen-year period and $3.0 million in trade name which we amortized over a two year period. The amortization expense for the Clarient intangible assets are included in general and administrative expense in the consolidated statements of operations. The trade name has been fully amortized as of December 31, 2017.
Path Logic
In July 2014, we acquired Path Logic and recorded $1.93 million in customer relationships as an intangible asset. We were amortizing these customer relationships over a thirteen-year period. The amortization expense was included in general and administrative expense in the consolidated statements of operations.
In the fourth quarter of 2016, due to declining volumes and revenues from customer losses, we engaged a valuation expert to perform an impairment assessment of the Path Logic customer relationships intangible asset. Based on the results of this assessment, we determined that the fair value of the Path Logic customer list was less than the carrying amount and the assets were fully impaired. An impairment loss was reported for the unamortized balance of the asset in the amount of approximately $1.6 million. On August 1, 2017, Path Logic was sold and a loss on the sale of approximately $1.1 million was recorded in the third quarter of 2017.
56
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
In August 2017, we acquired a customer list and recorded $4.1 million in intangible assets comprised of customer relationships. The amortization expense is included in general and administrative expense in the consolidated statements of operations.
License Agreement
In January 2012, we acquired approximately $3.0 million of intangible assets related to our Master License Agreement with Health Discovery Corporation (“HDC”) pursuant to which we were granted an exclusive worldwide license to utilize 84 issued and pending patents to develop and commercialize LDTs and other products relating to hematopoietic and solid tumor cancers. The licensed intellectual property and know-how relates to support vector machine, recursive feature elimination, fractal genomic modeling and other pattern recognition technology as well as certain patents relating to digital image analysis, biomarker discovery, and gene and protein-based diagnostic, prognostic, and predictive testing.
In the fourth quarter of 2016, the Company considered several factors in making a determination that the HDC assets were fully impaired. Key factors considered were the lack of revenues to date and the disputed license termination notification received from HDC. Based on this analysis, the Company determined that the assets were fully impaired and an impairment loss was recorded for the unamortized balance of these assets in the amount of $1.9 million.
Stock Based Compensation
The Company recognizes compensation costs for all share-based payment awards made to employees, non-employee contracted physicians and directors based upon the awards’ initial grant-date fair value. The fair value of awards to non-employees are then marked-to-market each reporting period until vesting criteria are met.
For stock options, the Company uses a trinomial lattice option-pricing model to estimate the fair value of stock option awards, and recognizes compensation cost on a straight-line basis over the awards’ requisite service periods for employees and variably for non-employees due to the marked-to-market adjustments at the end of each reporting period. The Company’s periodic expense is adjusted for actual forfeitures.
See Note B and Note K in the Consolidated Financial Statements included in this Annual Report for more information regarding the assumptions used in our valuation of stock-based compensation.
Deferred Taxes
Our accounting for deferred tax consequences represents our best estimate of future events that can be appropriately reflected in accounting estimates. Changes in existing tax laws, regulations, rates and future operating results may impact the amount of deferred tax liabilities and deferred tax assets over time. We allocate our deferred tax asset and liabilities based on the classification of the item creating the deferred or when we believe the deferred will be realized if there is no corresponding item.
Management assesses the available positive and negative evidence to estimate if sufficient future taxable income will be generated to use the existing deferred tax assets. As of December 31, 2017 and 2016 we did not record a valuation allowance as management determined that sufficient positive evidence exists to conclude that it is more likely than not that deferred taxes are realizable.
57
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Results of Operations for the year ended December 31, 2017 as compared with the year ended December 31, 2016
The following table presents the condensed consolidated statements of operations as a percentage of revenue:
|
|
|
For the years ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
NET REVENUE |
|
|
100.0 |
% |
|
|
100.0 |
% |
|
Cost of revenue |
|
|
53.5 |
% |
|
|
54.8 |
% |
|
GROSS PROFIT |
|
|
46.5 |
% |
|
|
45.2 |
% |
|
OPERATING EXPENSES: |
|
|
|
|
|
|
|
|
|
General and administrative |
|
|
34.3 |
% |
|
|
31.0 |
% |
|
Research and development |
|
|
1.4 |
% |
|
|
1.9 |
% |
|
Sales and marketing |
|
|
9.5 |
% |
|
|
9.8 |
% |
|
Impairment charges |
|
|
0.0 |
% |
|
|
1.4 |
% |
|
Total operating expenses |
|
|
45.2 |
% |
|
|
44.1 |
% |
|
INCOME FROM OPERATIONS |
|
|
1.3 |
% |
|
|
1.1 |
% |
|
Interest expense, net |
|
|
2.1 |
% |
|
|
4.1 |
% |
|
Other expense |
|
|
0.5 |
% |
|
|
— |
|
|
Net (loss) before income taxes |
|
|
(1.3 |
)% |
|
|
(3.0 |
)% |
|
Income tax (benefit) |
|
|
(1.0 |
)% |
|
|
(0.7 |
)% |
|
NET (LOSS) |
|
|
(0.3 |
)% |
|
|
(2.3 |
)% |
Revenue
Clinical and Pharma Services revenue for the periods presented are as follows ($ in thousands):
|
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
% Change |
|
|||
|
Net revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical Services |
|
$ |
231,748 |
|
|
$ |
222,015 |
|
|
|
4.4 |
% |
|
Pharma Services |
|
|
26,863 |
|
|
|
22,068 |
|
|
|
21.7 |
% |
|
Total Revenue |
|
$ |
258,611 |
|
|
$ |
244,083 |
|
|
|
6.0 |
% |
Consolidated revenues increased $14.5 million, or 6%, year-over-year. Growth in our clinical segment year-over-year, excluding revenue attributable to Path Logic which was sold on August 1, 2017, was $13.4 million, or 6.2%. Testing volumes also increased in our clinical segment by approximately 16.7% year-over-year. The increases in revenue and volume were largely due to strong growth in molecular and histology testing as well as growth in immuno-histochemistry tests due to demand for the PD-L1 test as a result of the FDA approving Pembrolizumab (Keytruda) in October 2016 as first-line treatment for PD-L1 positive non-small cell lung cancer. We have also seen accelerating growth in flow cytometry and FISH during the second half of the year. While revenues increased year over year, we believe the impact of Hurricanes Harvey and Irma depressed our revenues by approximately $1.0 million in the third quarter of 2017.
During 2017, our sales team finished the Clarient integration-related activities, which distracted them from their efforts to sell new business earlier in the year. The sales team is now re-focused on growth as evidenced by our fourth quarter revenue growth of 9.8% vs. the prior year (excluding the impact from the sale of PathLogic). This was our highest quarterly revenue growth during 2017.
Pharma Services revenue increased approximately $4.8 million, or 21.7%, year-over-year. In addition, our backlog of signed contracts has continued to grow from $36.4 million as of December 31, 2016 to $66.5 million as of December 31, 2017. We define backlog as the stated amount of signed contracts for active projects less
58
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
contingencies and cancellations. We expect this backlog to result in higher revenues in future years. We also expect to see growth in our Pharma Services segment due to our international expansion into Rolle, Switzerland as well as our increased capacity in our Houston, Texas facility. We expect this expansion to be complete in early April of 2018.
The following table shows clinical revenue, cost of revenue, requisitions received and tests performed for the years ended December 31, 2017 and 2016. This data excludes tests performed for Pharma customers and tests performed by Path Logic, which was sold on August 1, 2017. Testing revenue and cost of revenue are presented in thousands below:
|
|
|
December 31, |
|
|
|
|
|
|||||
|
|
|
2017 |
|
|
2016 |
|
|
% Change |
|
|||
|
Requisitions received (cases) |
|
|
394,520 |
|
|
|
361,220 |
|
|
|
9.2 |
% |
|
Number of tests performed |
|
|
657,394 |
|
|
|
563,132 |
|
|
|
16.7 |
% |
|
Average number of tests/requisition |
|
|
1.67 |
|
|
|
1.56 |
|
|
|
6.9 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total clinical genetic testing revenue |
|
$ |
228,078 |
|
|
$ |
214,708 |
|
|
|
6.2 |
% |
|
Average revenue/requisition |
|
$ |
578 |
|
|
$ |
594 |
|
|
|
(2.7 |
%) |
|
Average revenue/test |
|
$ |
347 |
|
|
$ |
381 |
|
|
|
(9.0 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenue |
|
$ |
117,839 |
|
|
$ |
113,373 |
|
|
|
3.9 |
% |
|
Average cost/requisition |
|
$ |
299 |
|
|
$ |
314 |
|
|
|
(5.2 |
%) |
|
Average cost/test |
|
$ |
179 |
|
|
$ |
201 |
|
|
|
(10.9 |
%) |
We continue to realize growth in clinical revenue, which we believe is the direct result of our efforts to innovate by developing and maintaining one of the most comprehensive cancer testing menus in the industry. Our broad test menu enables our sales teams to identify opportunities for increasing revenues from existing clients and allows us to gain market share from competitors. New molecular and immuno-histochemistry tests such as Micro Satellite Instability, DNA Mismatch Repair, PD1 and PD-L1 have continued to show solid growth and have increased our volume and revenue growth. We believe the field of immuno-therapy will continue to show substantial growth in coming years and our ability to offer multi-modality testing in one lab will allow us to capitalize on this increased demand.
Average revenue per test decreased year-over-year, primarily due to the change in test mix, specifically the increase in PD-L1 testing which has a lower average unit price (“AUP”) than our overall Company AUP. Additionally, revenue per test decreased as a result of the 2017 Medicare Physician Fee Schedule, which reduced Medicare Flow Cytometry reimbursement by 19%, and the combination of Clarient and NeoGenomics insurance contracts as several contracts were amended or renegotiated during 2017.
PathLogic was sold on August 1, 2017 as has been excluded from the above table for comparative purposes. During the seven months of ownership in 2017 NeoGenomics recorded revenue from PathLogic of $3.7 million. During twelve months of ownership in 2016, NeoGenomics recorded revenue from PathLogic of $7.3 million.
Cost of Revenue and Gross Margin
These decreases to our average revenue per test were offset by our higher volumes and 10.9% reduction in cost per test. The cost per test reductions were partially a result of the change in test mix, specifically the higher mix of lower cost histology tests. In addition, we continue to have success in reducing costs in the laboratory as synergies are being realized from the consolidation of our Irvine and Aliso Viejo, California laboratories. Our laboratory teams also made significant progress during 2017 lowering of our supplies costs and improving the efficiency of our medical technologists. We have also seen a reduction in send-out costs, as it is unlikely that we would need to send a test to another laboratory, due to our extensive test menu.
59
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Cost of revenue includes payroll and payroll-related costs for performing tests, depreciation of laboratory equipment, rent for laboratory facilities, laboratory reagents, probes and supplies, and delivery and courier costs relating to the transportation of specimens to be tested.
Clinical and Pharma Services cost of revenue and gross profit metrics for the periods presented are as follows ($ in thousands):
|
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
% Change |
|
|||
|
Cost of revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical Services |
|
$ |
121,785 |
|
|
$ |
120,437 |
|
|
|
1.1 |
% |
|
Pharma Services |
|
|
16,510 |
|
|
|
13,267 |
|
|
|
24.4 |
% |
|
Total Cost of Revenue |
|
$ |
138,295 |
|
|
$ |
133,704 |
|
|
|
3.4 |
% |
|
Cost of revenue as a % of revenue |
|
|
53.5 |
% |
|
|
54.8 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross Profit: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical Services |
|
$ |
109,963 |
|
|
$ |
101,578 |
|
|
|
8.3 |
% |
|
Pharma Services |
|
|
10,353 |
|
|
|
8,801 |
|
|
|
17.6 |
% |
|
Total Gross Profit |
|
$ |
120,316 |
|
|
$ |
110,379 |
|
|
|
|
|
|
Gross Profit Margin |
|
|
46.5 |
% |
|
|
45.2 |
% |
|
|
|
|
For 2017, consolidated cost of revenue as a percentage of revenue was 53.5% compared to 54.8%, in 2016, and 2017 gross profit margin was 46.5% compared to 45.2% in 2016. This 130 basis point improvement primarily reflects processing efficiencies on increased test volumes, including limited laboratory staffing increases, a reduction in costs per test, and the realization of certain synergies that we anticipated from the acquisition of Clarient and the combination of our two southern California labs.
General and Administrative Expenses
General and administrative expenses consist of employee-related costs (salaries, fringe benefits, and stock based compensation expense) for our billing, finance, human resources, information technology and other administrative personnel. We also allocate professional services, facilities expense, IT infrastructure costs, bad debt expense, depreciation, amortization and other administrative-related costs to general and administrative expenses.
Consolidated general and administrative expenses for the periods presented are as follows ($ in thousands):
|
|
|
For the years ended December 31. |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
2017 |
|
|
2016 |
|
|
$ Change |
|
|
% Change |
|
||||
|
General and administrative |
|
$ |
88,755 |
|
|
$ |
75,782 |
|
|
$ |
12,973 |
|
|
|
17.1 |
% |
|
General and administrative as a % of revenue |
|
|
34.3 |
% |
|
|
31.0 |
% |
|
|
|
|
|
|
|
|
For fiscal 2017, general and administrative expenses increased $13.0 million, or 330 basis points, compared to 2016, primarily reflecting increases in bad debt, professional fees, and personnel fees including stock based compensation, and depreciation and amortization expense.
Bad debt expense for the year ended December 31, 2017 increased by approximately $6.8 million compared to the same period in 2016. Bad debt as a percentage of revenue was 7.2% in 2017, or a 230 basis point increase, compared to 4.9% in 2016. The increase in bad debt is primarily related to changes in payer dynamics, including preauthorization denials as well as increased denials for next generation sequencing tests and disease specific multi-gene panels. In addition, there was a significant impact from the integration of Clarient into our billing system, which began in July of 2016. Billings of the legacy Clarient billing system have now been either fully collected or written off. The performance of our billing team was also impacted by the integration as well as our overall test growth, which ultimately contributed to certain receivables not being collected and increased bad debt expense.
60
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Professional fees for the year ended December 31, 2017 increased by approximately $2.4 million, or 80 basis points, when compared to the same period in 2016, primarily due to fees in 2017 related to the Pharma Services facility in Rolle, Switzerland, an increase in legal reserves related to a lawsuit brought against Clarient.
Depreciation and amortization expenses for the year ended December 31, 2017 increased by approximately $1.9 million, or 50 basis points, when compared to the same period in 2016, primarily reflecting increases in capital expenditures over the last two years.
Payroll expenses for the year ended December 31, 2017 increased by approximately $1.5 million when compared to the same period in 2016, primarily reflecting additional staff hired for certain functions such as billing, IT and accounts payable. As a percentage of revenue, payroll expenses decreased by 10 basis points, reflecting leverage on increased revenues.
We expect our general and administrative expenses to increase as we add personnel and equity compensation expenses, increase our billing and collections activities, incur additional expenses associated with the expansion of our facilities and backup systems, incur additional bad debt expense as sales increase and as we continue to expand our physical infrastructure to support our anticipated growth. A significant portion of our stock based compensation is for non-employee options, which are subject to variable accounting, and our expenses will fluctuate based on the performance of our common stock. A rise in the price of our stock will increase our stock compensation expense, and a decline in our stock price will reduce this expense. However, we anticipate that general and administrative expenses as a percentage of consolidated revenue will decrease over the coming years as we continue to grow.
Research and Development Expenses
Research and development expenses relate to cost of developing new proprietary and non-proprietary genetic tests, including payroll and payroll-related costs, maintenance and depreciation of laboratory equipment, laboratory supplies (reagents), outside consultants and experts assisting our research and development team.
Stock based compensation recorded in research and development expenses relates to unvested equity awards granted to non-employee physicians. Because portions of the vesting requirements have not been met, the amount of expense is re-measured at the end of each accounting period. We expect our research and development expenses to fluctuate in future periods because of increases or decreases in our stock price and the corresponding stock based compensation expense for non-employee stock options. Increases in our stock price result in additional expense and decreases in our stock price can result in recovery of previously recorded expense.
Consolidated research and development expense for the periods presented are as follows ($ in thousands):
|
|
|
For the years ended December 31. |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
2017 |
|
|
2016 |
|
|
$ Change |
|
|
% Change |
|
||||
|
Research and development |
|
$ |
3,636 |
|
|
$ |
4,649 |
|
|
$ |
(1,013 |
) |
|
|
(21.8 |
%) |
|
Research and development as a % of revenue |
|
|
1.4 |
% |
|
|
1.9 |
% |
|
|
|
|
|
|
|
|
Research and development expense for the year ended December 31, 2017 decreased $1.0 million, or 50 basis points, when compared to the same period in 2016, primarily reflecting a decrease in contract labor and amortization expense, partially offset by an increase in payroll and payroll-related costs. The decrease in amortization expense reflected Health Discovery Corporation license agreements, which were being amortized as intangible assets in 2016 but were fully impaired in the fourth quarter of 2016.
We anticipate research and development expenditures will increase over time as we continue to invest in innovation projects and bringing new tests to market.
61
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Sales and marketing expenses are primarily attributable to employee-related costs including sales management, sales representatives, sales and marketing consultants, marketing, and customer service personnel. Costs also include various marketing-related costs such as attending trade shows, advertising and maintaining our website.
Consolidated sales and marketing expenses for the periods presented are as follows ($ in thousands):
|
|
|
For the years ended December 31. |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
2017 |
|
|
2016 |
|
|
$ Change |
|
|
% Change |
|
||||
|
Sales and marketing |
|
$ |
24,543 |
|
|
$ |
23,910 |
|
|
$ |
633 |
|
|
|
2.6 |
% |
|
Sales and marketing as a % of revenue |
|
|
9.5 |
% |
|
|
9.8 |
% |
|
|
|
|
|
|
|
|
For 2017, sales and marketing expenses as a percentage of revenue improved by 30 basis points compared to 2016, primarily reflecting leverage of our sales team on increased volumes and revenue in 2017. The $0.6 million increase in sales and marketing expenses primarily reflects higher commissions in line with increased revenue. We expect higher commissions expense in the coming quarters as the sales representatives’ focus on generating new business and continuing to increase revenue. In addition, we increased our investment in marketing-related activities in 2017, including trade shows and online marketing. We expect our sales and marketing expenses over the long term to increase as our test volumes increase, but to remain stable as a percentage of our overall sales.
Interest Expense, net and Other Income
Interest expense, net is comprised of interest incurred on our term debt, revolving credit facility and our capital lease obligations, offset by the interest income we earn on cash deposits. Interest expense, net decreased $4.5 million for the year ended December 31, 2017 compared to the same period in 2016, primarily reflecting the significantly lower borrowing rate on the Loan Agreement entered into in December of 2016. In addition, we have entered into a swap agreement to hedge a significant portion of the interest on our term loan; however, part of that loan is not hedged, nor is our revolving credit facility and they will continue to fluctuate as the LIBOR rates change.
Net (Loss)
The following table provides the net loss for each period along with the computation of basic and diluted net income per share (in thousands, except per share amounts):
|
|
|
Years Ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
NET (LOSS) ATTRIBUTABLE TO COMMON STOCKHOLDERS |
|
$ |
(11,393 |
) |
|
$ |
(30,397 |
) |
|
|
|
|
|
|
|
|
|
|
|
Basic weighted average common shares outstanding |
|
|
79,426 |
|
|
|
77,542 |
|
|
Effect of potentially dilutive securities |
|
|
— |
|
|
|
— |
|
|
Diluted weighted average shares outstanding |
|
|
79,426 |
|
|
|
77,542 |
|
|
|
|
|
|
|
|
|
|
|
|
Basic net (loss) per common share |
|
$ |
(0.14 |
) |
|
$ |
(0.39 |
) |
|
Diluted net (loss) per share |
|
$ |
(0.14 |
) |
|
$ |
(0.39 |
) |
Non-GAAP Measures
Use of non-GAAP Financial Measures
Our financial results are provided in accordance with accounting principles generally accepted in the United States of America (GAAP) and using certain non-GAAP financial measures. Management believes that presentation of operating results using non-GAAP financial measures provides useful supplemental information to investors and
62
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
facilitates the analysis of the Company’s operating results and comparison of operating results across reporting periods and between entities. Management also uses non-GAAP financial measures for financial and operational decision making, planning and forecasting purposes and to manage our business. Management believes that Adjusted EBITDA is a key metric for our business because it is used by our lenders in the calculation of our debt covenants. Management also believes that these non-GAAP financial measures enable investors to evaluate our operating results and future prospects in the same manner as management. The non-GAAP financial measures do not replace the presentation of GAAP financial results and should only be used as a supplement to, and not as a substitute for, our financial results presented in accordance with GAAP. There are limitations inherent in non-GAAP financial measures because they exclude charges and credits that are required to be included in a GAAP presentation, and do not therefore present the full measure of our recorded costs against its net revenue. In addition, our definition of the non-GAAP financial measures below may differ from non-GAAP measures used by other companies.
Definitions of non-GAAP measures
Non – GAAP EBITDA
We define Non-GAAP “EBITDA” as net income from continuing operations before: (i) interest expense, (ii) tax expense and (iii) depreciation and amortization expense.
Non – GAAP Adjusted EBITDA
We define Non-GAAP “Adjusted EBITDA” as net income from continuing operations before: (i) interest expense, (ii) tax expense, (iii) depreciation and amortization expense, (iv) non-cash, stock-based compensation and warrant amortization expense, and if applicable in a reporting period (v) transaction expenses related to acquisitions and potential acquisitions, (vi) non-cash impairments of intangible assets (vii) debt financing costs and (viii)other significant non-recurring or non-operating (income) or expenses.
Basis for Non-GAAP Adjustments
Our basis for excluding certain expenses from GAAP financial measures, are outlined below:
|
|
• |
Interest expense – The capital structure of companies significantly affects the amount of interest expense incurred. This expense can vary significantly between periods and between companies. In order to compare performance between periods and companies that have different capital structures and thus different levels of interest obligations, NeoGenomics excludes this expense. |
|
|
• |
Income tax expense (benefit) – The tax positions of companies can vary because of their differing abilities to take advantage of tax benefits and because of the tax policies of the jurisdictions in which they operate. As a result, effective tax rates and the provision for income taxes can vary considerably among companies. In addition, the income tax benefit in 2017 includes a one-time tax benefit specifically related to the passing of the Tax Cut and Jobs Act, which was signed into law in December 2017. In order to compare performance between companies, NeoGenomics excludes this expense (benefit). |
|
|
• |
Depreciation expense – Companies utilize assets with different useful lives and use different methods of both acquiring and depreciating these assets. These differences can result in considerable variability in the costs of productive assets and the depreciation and amortization expense among companies. In order to compare performance between companies, NeoGenomics excludes this expense. |
|
|
• |
Amortization expense – The intangible assets that give rise to this amortization expense relate to acquisitions, and the amounts allocated to such intangible assets and the terms of amortization vary by acquisition and type of asset. NeoGenomics excludes these items to provide a consistent basis for comparing operating results across reporting periods, pre and post-acquisition. |
63
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
|
|
• |
Transaction expenses relating to acquisitions - We incurred significant expenses in connection with our recent acquisition of Clarient. The inclusion of these costs consisting primarily of transaction costs as well as outside consultants and related services result in considerable variability between periods. In order to compare across periods on a consistent basis we believe it is useful to exclude these expenses. |
|
|
• |
Debt financing costs – The amount and frequency of debt financing costs are significantly impacted by the timing and size of debt financing transactions. The amount and frequency of such charges are not consistent and therefore without adjusting for these costs, the Company believes it would not allow for consistent comparison between reporting periods. |
|
|
• |
Moving expenses – These expenses include costs associated with the move of our Irvine, California facility into our Aliso Viejo facility and restoring the Irvine facility back to its original condition at the end of the lease term. We are adjusting for these costs in Adjusted EBITDA as the move was the direct result of the Clarient acquisition and will not be an annually recurring item. Without adjusting for these expenses, the Company believes it would be difficult to compare financial results from operations across reporting periods on a consistent basis. |
|
|
• |
Non-cash impairments - We exclude these impairments in our calculation of Adjusted EBITDA, as they entail no outlay of cash and reduce the comparability of financial results between periods. |
We believe that EBITDA and Adjusted EBITDA provide more consistent measures of operating performance between entities and across reporting periods by excluding cash and non-cash items of expense that can vary significantly between companies. In addition, Adjusted EBITDA is a metric that is used by our lenders in the calculation of our debt covenants. Adjusted EBITDA also assists investors in performing analyses that are consistent with financial models developed by independent research analysts.
EBITDA and Adjusted EBITDA (as defined by us) are not measurements under GAAP and may differ from non-GAAP measures used by other companies. We believe there are limitations inherent in non-GAAP financial measures such as EBITDA and Adjusted EBITDA because they exclude a variety of charges and credits that are required to be included in a GAAP presentation, and do not therefore present the full measure of NeoGenomics recorded costs against its net revenue. Accordingly, we encourage investors to consider both non-GAAP results together with GAAP results in analyzing our financial performance.
64
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
The following is a reconciliation of GAAP net loss to Non-GAAP EBITDA and Adjusted EBITDA for the years ending December 31, 2017 and 2016 ($ in thousands):
|
|
|
For the years ended December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
NET (LOSS) (per GAAP) |
|
$ |
(846 |
) |
|
$ |
(5,723 |
) |
|
|
|
|
|
|
|
|
|
|
|
Adjustments to net income: |
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
5,540 |
|
|
|
9,998 |
|
|
Amortization of intangibles |
|
|
6,995 |
|
|
|
7,272 |
|
|
Income tax (benefit) |
|
|
(2,635 |
) |
|
|
(1,701 |
) |
|
Depreciation of property and equipment |
|
|
15,596 |
|
|
|
15,937 |
|
|
EBITDA (non-GAAP) |
|
|
24,650 |
|
|
|
25,783 |
|
|
|
|
|
|
|
|
|
|
|
|
Further Adjustments to EBITDA: |
|
|
|
|
|
|
|
|
|
Facility moving expenses and other adjustments |
|
|
620 |
|
|
|
- |
|
|
Impairment charges |
|
|
- |
|
|
|
3,464 |
|
|
Loss on sale of business |
|
|
1,058 |
|
|
|
- |
|
|
Non-cash stock-based compensation |
|
|
6,441 |
|
|
|
5,438 |
|
|
ADJUSTED EBITDA (non-GAAP) |
|
$ |
32,769 |
|
|
$ |
34,685 |
|
|
Adjusted EBITDA as % of Revenue |
|
|
12.7 |
% |
|
|
14.2 |
% |
Results of Operations for the year ended December 31, 2016 as compared with the year ended December 31, 2015
The following table presents the condensed consolidated statements of operations as a percentage of revenue:
|
|
|
For the years ended December 31. |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
NET REVENUE |
|
|
100.0 |
% |
|
|
100.0 |
% |
|
Cost of revenue |
|
|
54.8 |
% |
|
|
56.2 |
% |
|
GROSS PROFIT |
|
|
45.2 |
% |
|
|
43.8 |
% |
|
OPERATING EXPENSES: |
|
|
|
|
|
|
|
|
|
General and administrative |
|
|
31.0 |
% |
|
|
33.7 |
% |
|
Research and development |
|
|
1.9 |
% |
|
|
4.2 |
% |
|
Sales and marketing |
|
|
9.8 |
% |
|
|
11.6 |
% |
|
Impairment charges |
|
|
1.4 |
% |
|
|
— |
|
|
Total operating expenses |
|
|
44.1 |
% |
|
|
49.5 |
% |
|
INCOME (LOSS) FROM OPERATIONS |
|
|
1.1 |
% |
|
|
(5.6 |
)% |
|
Interest expense, net |
|
|
4.1 |
% |
|
|
0.9 |
% |
|
Other income |
|
|
— |
|
|
|
2.0 |
% |
|
Net loss before income taxes |
|
|
3.0 |
% |
|
|
4.5 |
% |
|
Income taxes benefit |
|
|
0.7 |
% |
|
|
2.0 |
% |
|
NET LOSS |
|
|
2.3 |
% |
|
|
2.5 |
% |
65
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Clinical and Pharma Services revenue for the periods presented are as follows ($ in thousands):
|
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
% Change |
|
|||
|
Net revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical Services |
|
$ |
222,015 |
|
|
$ |
98,595 |
|
|
|
125.2 |
% |
|
Pharma Services |
|
|
22,068 |
|
|
|
1,207 |
|
|
|
1728.3 |
% |
|
Total Revenue |
|
$ |
244,083 |
|
|
$ |
99,802 |
|
|
|
144.6 |
% |
Clinical revenue above includes Path Logic revenue of $7.3 million and $8.1 million for the years ended December 31, 2016 and 2015, respectively. Our clinical revenue, excluding Path Logic, grew by $124.2 million or 137.2% year-over-year. This growth is primarily the result of a broad based increase in the number of new clients due to the Clarient acquisition, as is also evidenced by the 159.5% increase in case volume. The acquisition has enabled us to expand into geographical areas we previously did not have a presence which has added to our client base and revenues. In addition, the increase in revenues are a result of our efforts to innovate by developing one of the most comprehensive molecular testing menus in the industry. Our testing menu has allowed us to up-sell tests to Clarient customers that they previously had to order from other laboratories, which is also driving our revenues and growth.
In addition, the increase in revenues are a result of our efforts to innovate by developing one of the most comprehensive molecular testing menus in the industry. For example, our comprehensive testing menu has allowed us to offer tests to Clarient customers that they previously had to order from other laboratories. New tests and innovation, such as PD-L1 testing, also contributed to our growth.
In the fourth quarter of 2016, we saw a significant increase in the demand for the PD-L1 and believe we are currently a market leader in this important immuno-oncology test offering.
Average revenue per requisition as well as average revenue per test decreased in 2016 as compared to 2015. These decreases were largely due to product mix changes, specifically the increase in PD-L1 testing which has a lower unit price. These decreases were offset by our higher volumes as well as our reduction in cost per test.
During 2016, we completed the integration of all Clarient clients to the NeoGenomics test menu. This was a significant task and a distraction for our sales team which including training the Clarient clients on the new ordering system as well as educating them on the new test menu.
Our Pharma Services business reported revenue in 2016 of $22.1 million, up from $1.2 million in 2015. This was due to the inclusion of Clarient’s results as they had a much larger Pharmaceutical Services business than legacy NeoGenomics before the acquisition. We are investing in this business and believe it will be a significant growth driver for us in future periods as the market for oncology clinical trials continues to expand.
66
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
The following table shows clinical genetic testing revenue, cost of revenue, requisitions received and tests performed for the years ended December 31, 2016 and 2015. This data excludes tests performed for Pharma Services and tests performed by Path Logic. Testing revenue and cost of revenue are presented in thousands below:
|
|
|
December 31, |
|
|
|
|
|
|||||
|
|
|
2016 |
|
|
2015 |
|
|
% Change |
|
|||
|
Requisitions received (cases) |
|
|
361,220 |
|
|
|
139,195 |
|
|
|
159.5 |
% |
|
Number of tests performed |
|
|
563,132 |
|
|
|
221,191 |
|
|
|
154.6 |
% |
|
Average number of tests/requisition |
|
|
1.56 |
|
|
|
1.59 |
|
|
|
(1.9 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total clinical genetic testing revenue |
|
$ |
214,708 |
|
|
$ |
90,506 |
|
|
|
137.2 |
% |
|
Average revenue/requisition |
|
$ |
594 |
|
|
$ |
650 |
|
|
|
(8.6 |
%) |
|
Average revenue/test |
|
$ |
381 |
|
|
$ |
409 |
|
|
|
(6.8 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenue |
|
$ |
113,373 |
|
|
$ |
48,783 |
|
|
|
132.4 |
% |
|
Average cost/requisition |
|
$ |
314 |
|
|
$ |
350 |
|
|
|
(10.3 |
%) |
|
Average cost/test |
|
$ |
201 |
|
|
$ |
221 |
|
|
|
(9.0 |
%) |
Cost of Revenue and Gross Margin
Cost of revenue includes payroll and payroll related costs for performing tests, depreciation of laboratory equipment, rent for laboratory facilities, laboratory reagents, probes and supplies, and delivery and courier costs relating to the transportation of specimens to be tested.
Cost of revenue year-over-year increased by approximately 132%, primarily due to our increase in testing volume from the Clarient acquisition. As a percentage of revenue, costs declined slightly. We have begun to realize the benefits of our increased volumes and were able to reduce cost per test year-over-year by 9.0%. We will continue to realize the benefit of scale as we route higher volumes through our existing laboratories, especially as we combine two of our California laboratories in early 2017.
Average cost per requisition also decreased in 2016 as compared to 2015, which is attributable to changes in product mix as well as operating efficiencies. Our best practice teams have been working closely with our information technology team to re-design the laboratory information system. We expect this to increase efficiency in the labs and improve our processes. We continue to focus on improving our laboratory operations in order to drive further improvements in our cost per test. We believe that we have only begun to achieve the potential synergies from the Clarient acquisition and expect to further reduce cost per test in 2017.
67
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
The Clinical and Pharma Services cost of revenue and gross profit metrics for the periods presented are as follows ($ in thousands):
|
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
|
2016 |
|
|
2015 |
|
|
% Change |
|
|||
|
Cost of revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical Services |
|
$ |
120,437 |
|
|
$ |
55,802 |
|
|
|
115.8 |
% |
|
Pharma Services |
|
|
13,267 |
|
|
|
244 |
|
|
|
5337.3 |
% |
|
Total Cost of Revenue |
|
$ |
133,704 |
|
|
$ |
56,046 |
|
|
|
138.6 |
% |
|
Cost of revenue as a % of revenue |
|
|
54.8 |
% |
|
|
56.2 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross Profit: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical Services |
|
$ |
101,578 |
|
|
$ |
42,793 |
|
|
|
137.4 |
% |
|
Pharma Services |
|
|
8,801 |
|
|
|
963 |
|
|
|
813.9 |
% |
|
Total Gross Profit |
|
$ |
110,379 |
|
|
$ |
43,756 |
|
|
|
|
|
|
Gross Profit Margin |
|
|
45.2 |
% |
|
|
43.8 |
% |
|
|
|
|
General and Administrative Expenses
General and administrative expenses relate to billing, bad debts, finance, human resources, information technology and other administrative functions. They primarily consist of employee related costs (such as salaries, fringe benefits, and stock-based compensation expense), professional services, facilities expense, and depreciation and administrative-related costs allocated to general and administrative expenses.
Consolidated general and administrative expenses for the periods presented are as follows ($ in thousands):
|
|
|
For the years ended December 31. |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
2016 |
|
|
2015 |
|
|
$ Change |
|
|
% Change |
|
||||
|
General and administrative |
|
$ |
75,782 |
|
|
$ |
33,631 |
|
|
$ |
42,151 |
|
|
|
125.3 |
% |
|
General and administrative as a % of revenue |
|
|
31.0 |
% |
|
|
33.7 |
% |
|
|
|
|
|
|
|
|
General and administrative expenses increased for the year ended December 31, 2016 as compared to the year ended December 31, 2015, while as a percentage of revenue there was a slight decrease. These increases in general and administrative expenses were primarily due to the integration of Clarient and the additional resources necessary to manage the growth of the Company and the increased volume of testing. The majority of this increase was in the line items of payroll and payroll related expenditures and bad debt expense. In addition, $2.1 million of the increase is attributable to non-cash stock based compensation expense as a result of new options issued in 2016 and the increase in NeoGenomics stock price during 2016 which impacts stock options issued to non-employees, as awards to non-employees that are not vested require marked-to-market adjustments each reporting period.
A significant portion of our stock based compensation is for non-employee options which are subject to variable accounting, and our expenses will fluctuate based on the performance of our common stock. A rise in the price of our stock will increase our stock compensation expense, and a decline in our stock price will reduce this expense.
Bad debt expense increased approximately $9.5 million to $11.9 million for the year ended December 31, 2016 as compared to the year ended December 31, 2015. As a percentage of revenue, bad debt expense was 4.9% for the period ended December 31, 2016 compared to 2.3% for the period ended December 31, 2015. This increase in bad debt expense is attributable to the inclusion of Clarient’s results, which had a historically higher bad debt rate than legacy NeoGenomics.
68
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Research and Development Expenses
Research and development, or R&D expenses relate to cost of developing new proprietary and non-proprietary genetic tests as well as costs related to our licensing agreement with Health Discovery Corporation. Expenses include amortization of the licensed technology, payroll and payroll related costs, maintenance and depreciation of laboratory equipment, laboratory reagents, probes and supplies.
Stock based compensation, recorded in research and development relates to unvested equity awards granted to a non-employee physician. Because portions of the vesting requirements have not been met, the amount of expense is re-measured at the end of each accounting period.
Consolidated research and development expense for the periods presented are as follows ($ in thousands):
|
|
|
For the years ended December 31. |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
2016 |
|
|
2015 |
|
|
$ Change |
|
|
% Change |
|
||||
|
Research and development |
|
$ |
4,649 |
|
|
$ |
4,198 |
|
|
$ |
451 |
|
|
|
10.7 |
% |
|
Research and development as a % of revenue |
|
|
1.9 |
% |
|
|
4.2 |
% |
|
|
|
|
|
|
|
|
Excluding stock based compensation of $789,000 and $1.2 million, research and development expense was approximately $3.9 million and $3.0 million for the years ended December 31, 2016 and 2015, respectively. The year over year variances in stock based compensation expense are directly related to the fluctuations in our stock price. The remaining increase of approximately 30% was due to increases in labor, contract labor and equipment related to the development of new tests.
Sales and Marketing
Sales and marketing expenses are primarily attributable to employee related costs including sales management, sales representatives, sales and marketing consultants, marketing, and customer service personnel. Costs also include various marketing related costs such as attending trade shows, advertising and maintaining our web site.
Consolidated sales and marketing expenses for the periods presented are as follows ($ in thousands):
|
|
|
For the years ended December 31. |
|
|
|
|
|
|
|
|
|
|||||
|
|
|
2016 |
|
|
2015 |
|
|
$ Change |
|
|
% Change |
|
||||
|
Sales and marketing |
|
$ |
23,910 |
|
|
$ |
11,562 |
|
|
$ |
12,348 |
|
|
|
106.8 |
% |
|
Sales and marketing as a % of revenue |
|
|
9.8 |
% |
|
|
11.6 |
% |
|
|
|
|
|
|
|
|
Sales and marketing expenses increased for the year ended December 31, 2016 as compared to the year ended December 31, 2015. The increase in sales and marketing expenses was the direct result of our significantly larger sales force due to the acquisition of Clarient. In addition, we had higher expenditures for advertising and marketing which were partly due to the larger company and also due to our re-branding efforts. The decrease in our sales and marketing expenditures as a percentage of revenues can be attributed to the synergies obtained as a result of the acquisition.
Interest Expense, net and Other Income
Interest expense, net primarily consists of the interest we incur on capital lease and debt obligations offset by the interest income we earn on cash deposits. Interest expense, net increased from $854 thousand for the year ended December 31, 2015 to approximately $10.0 million for the year ended December 31, 2016. The increase is almost entirely due to interest payments on the Term Loan Facility and revolving credit facility entered into in association
69
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
with the Clarient acquisition. As this financing was closed in December of 2015, there were minimal interest costs for this facility for the year ended December 31, 2015.
In March of 2016, we paid off the revolving credit facility and in December of 2016, we paid off the Term Loan Facility. We incurred debt termination costs of approximately $1.1 million and also recognized approximately $2.8 million associated with the write off of debt issuance costs; these expenses are included in interest expense on the consolidated statement of operations. A new borrowing facility, at a lower interest rate was put into place on December 22, 2016, the proceeds of which were used to pay off the debt issued for the Clarient acquisition, and to redeem $55.0 million worth of our Series A Preferred Stock.
Other income of $2.0 million was recorded in 2015 related to a one-time payment received upon the amendment of a laboratory services contract and elimination of the exclusivity requirement. We had no other income reported for the year ended December 31, 2016.
Net Loss
The following table provides the net loss for each period along with the computation of basic and diluted net loss per share for the year ended December 31, 2016 and 2015 (in thousands, except per share amounts):
|
|
|
For the years ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS |
|
$ |
(30,397 |
) |
|
$ |
(2,657 |
) |
|
|
|
|
|
|
|
|
|
|
|
Basic weighted average common shares outstanding |
|
|
77,542 |
|
|
|
60,526 |
|
|
Effect of potentially dilutive securities |
|
|
- |
|
|
|
- |
|
|
Diluted weighted average shares outstanding |
|
|
77,542 |
|
|
|
60,526 |
|
|
|
|
|
|
|
|
|
|
|
|
Basic net loss per common share |
|
$ |
(0.39 |
) |
|
$ |
(0.04 |
) |
|
Diluted net loss per common share |
|
$ |
(0.39 |
) |
|
$ |
(0.04 |
) |
The following is a reconciliation of GAAP net loss to Non-GAAP EBITDA and Adjusted EBITDA for the years ending December 31, 2016 and 2015 ($ in thousands):
|
|
|
For the years ended December 31, |
|
|||||
|
|
|
2016 |
|
|
2015 |
|
||
|
NET LOSS (per GAAP) |
|
$ |
5,723 |
|
|
$ |
2,535 |
|
|
|
|
|
|
|
|
|
|
|
|
Adjustments to Net Loss: |
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
9,998 |
|
|
|
854 |
|
|
Amortization of intangibles |
|
|
7,272 |
|
|
|
412 |
|
|
Income taxes (benefit) expense |
|
|
(1,701 |
) |
|
|
(1,954 |
) |
|
Depreciation of property and equipment |
|
|
15,937 |
|
|
|
6,730 |
|
|
EBITDA (non-GAAP) |
|
|
25,783 |
|
|
|
3,507 |
|
|
|
|
|
|
|
|
|
|
|
|
Further Adjustments to EBITDA: |
|
|
|
|
|
|
|
|
|
Acquisition related transaction expense |
|
|
- |
|
|
|
4,686 |
|
|
Impairment charges |
|
|
3,464 |
|
|
|
- |
|
|
Gain on contract amendment |
|
|
- |
|
|
|
(2,000 |
) |
|
Non-cash stock-based compensation |
|
|
5,438 |
|
|
|
3,479 |
|
|
ADJUSTED EBITDA (non-GAAP) |
|
$ |
34,685 |
|
|
$ |
9,672 |
|
|
Adjusted EBITDA as a % of revenue |
|
|
14.2 |
% |
|
|
9.7 |
% |
70
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Liquidity and Capital Resources
The following table presents a summary of our cash flows provided by (used in) operating, investing and financing activities for the years ended December 31, 2017, 2016 and 2015 as well as the period ending cash and cash equivalents and working capital (in thousands).
|
|
|
For the years ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 (2) |
|
|||
|
Net cash provided by (used in): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities |
|
$ |
18,037 |
|
|
$ |
21,477 |
|
|
$ |
6,393 |
|
|
Investing activities |
|
|
(13,690 |
) |
|
|
(6,501 |
) |
|
|
(75,155 |
) |
|
Financing activities |
|
|
(4,095 |
) |
|
|
(25,871 |
) |
|
|
58,493 |
|
|
Effects of foreign exchange rate changes on cash and cash equivalents |
|
|
44 |
|
|
|
- |
|
|
|
- |
|
|
Net increase (decrease) in cash and cash equivalents |
|
|
296 |
|
|
|
(10,895 |
) |
|
|
(10,269 |
) |
|
Cash and cash equivalents, beginning of period |
|
|
12,525 |
|
|
|
23,420 |
|
|
|
33,689 |
|
|
Cash and cash equivalents, end of period |
|
$ |
12,821 |
|
|
$ |
12,525 |
|
|
$ |
23,420 |
|
|
Working Capital (1), end of period |
|
$ |
49,898 |
|
|
$ |
40,712 |
|
|
$ |
42,302 |
|
|
(1) |
Defined as current assets less current liabilities. |
|
(2) |
Reflects the acquisition of Clarient in December 2015. |
Cash Flows from Operating Activities
During the year ended December 31, 2017, cash flows from operating activities were $18.0 million, a $3.4 million decrease compared to 2016. The decrease primarily reflects an increase in working capital of $9.2 million, partially offset by an increase in our provision for bad debt of $6.8 million. The increase in working capital primarily reflects an increase in our accounts receivable and a decrease in accounts payable, partially offset by increases in accrued expenses. Our receivables have increased over this period due to growth, as well as our higher Pharma Services DSOs. We did experience a delay in billing some Pharma Services clients in the fourth quarter which contributed to the higher Pharma DSO’s. We are paying our vendors more promptly which has contributed to the sharp reduction in Accounts Payable during 2017.
During the year ended December 31, 2016, our operating activities generated $15.1 million more cash than was generated for the year ended December 31, 2015. This increase in cash provided from operations was primarily the result of the increases in revenues due to our growth as a result of the Clarient acquisition.
Cash Flows from Investing Activities
During the year ended December 31, 2017, cash used in investing activities increased by $7.2 million compared to the same period in 2016. This increase was due to equipment purchases and building improvements, which were necessary to support our continued growth and efficiency. Specifically, we have remodeled and upgraded our laboratory facilities in Aliso Viejo, California, expanded our Houston, Texas facility, opened our Rolle, Switzerland laboratory, invested in additional laboratory equipment to accommodate our growth and updated existing equipment that was acquired with the purchase of Clarient. These investments have been made to help us increase our capacity to handle future growth. We have also invested in a new trade show booth as well as upgrades to our IT security environment and our next generation Laboratory Information System (LIS).
71
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
We acquired Clarient in December of 2015 and paid $73.8 million of cash at closing. This transaction significantly impacted cash flows from investing activities and is the primary reason for the decrease in cash used in investing activities between 2016 and 2015. In 2016, we received $1.0 million as the final working capital settlement from GE related to the acquisition of Clarient in December of 2015. In addition, we paid $7.5 million on capital purchases in 2016 compared to $2.2 million in 2015.
Cash Flows from Financing Activities
During the year ended December 31, 2017, cash flows from financing activities decreased by approximately $21.8 million compared to the same period in 2016. This decrease primarily reflects $55 million that was paid to redeem preferred stock and $20 million and $12.9 million in proceeds received on our Term Loan and Revolving Credit facilities, respectively, in 2016 that did not recur in 2017. Cash flows from financing activities for 2017 includes $5.0 million in advances on our revolving credit facility during the first quarter of 2017, partially offset by a $2.5 million repayment on our revolving credit facility during the third quarter of 2017. In addition, the change reflects $3.8 million in repayments on our term loan during 2017 through quarterly principal repayments. The 2016 revolving credit facility was originally used to finance the acquisition of Clarient.
During the year ended December 31, 2016, cash flows from financing activities changed by $84.4 million as compared to 2015. The cash used in financing activities during 2016 includes the $55 million that was paid to redeem the preferred stock as well as the $55 million that was paid when we terminated the Term Loan Facility entered into in December 2015, and $10 million that was repaid on the revolver in March of 2016. These amounts were offset by proceeds received from our new Term Loan Facility of $75 million, and $22.9 million that was borrowed on the new Revolving Credit Facility.
Credit Facility
During December of 2016, we entered into a new senior secured credit facility. In order to reduce our exposure to interest rate fluctuations on this floating rate debt obligation, we also entered into an interest rate swap agreement. For more information on this hedging instrument, see Note G to Consolidated Financial Statements herein. The interest rate swap agreement effectively converts a portion of our floating rate debt to a fixed obligation, thus reducing the impact of interest rate changes on future interest expense. We believe this strategy will enhance our ability to manage cash flow within our Company.
Liquidity Outlook
We had approximately $12.8 million in cash and cash equivalents as of December 31, 2017. In addition, we have a revolving credit facility which provides for up to $75 million in borrowing capacity of which at December 31, 2017, based on our level of Adjusted EBITDA, approximately $16.7 million was available. We believe that the cash on hand, available credit lines and positive cash flows generated from operations will provide adequate resources to meet our operating commitments and interest payments for at least the next 12 months from the issuance of these financial statements.
Our Series A Preferred Stock has certain restrictions that will result in the Company having to dedicate fifty percent of the net proceeds from any future equity raise, to redeeming shares of the Series A Preferred Stock until such time as all of the shares of Series A Preferred Stock have been redeemed. In addition, our Credit Agreement contains certain provisions beginning with the Annual Compliance Certificate for the fiscal year ended December 31, 2017, that would require a portion of the excess cash flow (as defined) to be repaid to our lenders. The debt repayment would be required five business days after the filing of our Annual Compliance Certificate. At December 31, 2017, no excess cash flow payment was due.
We are constructing a new facility in Houston, Texas which we anticipate to complete in the second quarter of 2018. The cost to complete the construction of this facility will be approximately $2.9 million which will be funded primarily through lease financing.
72
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Consulting Agreements
During the years ended December 31, 2017, 2016 and 2015, Steven C. Jones, a director of the Company, earned approximately $247,000, $263,000 and $261,500, respectively, for various consulting work performed in connection with his duties as an Executive Vice President and received reimbursement of incurred expenses. Mr. Jones also earned $31,912, $85,000 and $578,900 as payment of bonuses for the periods indicated above. The bonus earned for the year ended December 31, 2015 was comprised of $500,000 in recognition of the services provided in connection with the Company’s acquisition of Clarient, Inc. and the related financing. This amount was paid to Aspen Capital Advisors, LLC (“Aspen”) for which Mr. Jones is a managing director, pursuant to a consulting agreement entered into between Aspen and the Company on November 11, 2015. The remaining $78,900 was earned as part of a management incentive plan.
On May 25, 2017, the Company granted Mr. Jones 10,000 stock options to purchase shares of parent common stock. The options were granted at a price of $7.27 per share and had a weighted average fair market value of $2.47 per option. The options vest ratably over the next three years on each anniversary date. These options were accounted for as granted accounted for as granted to a Director of the Company. In addition, the Company granted Mr. Jones 8,667 shares of restricted common stock. Such restricted common stock vests ratably over each of the subsequent three quarters so long as he continues to serve as a member of the Board of Directors. The fair market value per share was deemed to be $63,009 or $7.27 per share, which was the closing price of Parent’s common stock on the day before the grant was approved by the compensation committee of the Board of Directors.
On April 20, 2016, the Company granted Mr. Jones 100,000 stock options to purchase shares of parent common stock. The options were granted at a price of $7.15 per share and had a weighted average fair market value of $2.50 per option. The options vest ratably over the next three years on each anniversary date. These options were accounted for as granted to a non-employee as they relate to his services to the Company as a consultant.
On May 4, 2015, the Company granted Mr. Jones 225,000 stock options to purchase shares of parent common stock. The options were granted at a price of $4.78 per share and had a weighted average fair market value of $1.80 per option. The options vest ratably over the next three years on each anniversary date. 10,000 of the options were accounted for as granted to a Director of the Company, consistent with similar grants at that time to other Directors. The remaining 215,000 stock options have been accounted for as granted to a non-employee as they relate to his services to the Company as a consultant.
On May 3, 2010, the Company entered into a consulting agreement (the “Consulting Agreement”) with Mr. Jones whereby Mr. Jones would continue to provide consulting services to the Company in the capacity of Executive Vice President of Finance. On May 3, 2010, the Company also entered into a warrant agreement with Mr. Jones and it issued a warrant to purchase 450,000 shares of the Company’s common stock, which were all vested as of December 31, 2016 and fully exercised at December 31, 2017.
73
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
On November 4, 2016, the Company entered into an amended and restated consulting agreement (the “Amended and Restated Consulting Agreement”) with Mr. Jones. The Amended and Restated Consulting Agreement has an initial term of November 4, 2016 through April 30, 2020, which initial term automatically renews for additional one year periods unless either party provides notice of termination at least three months prior to the expiration of the initial term or any renewal term. In addition, the Company has the right to terminate the Amended and Restated Consulting Agreement by giving written notice to Mr. Jones the year prior to the effective date of termination. Mr. Jones has the right to terminate the Amended and Restated Consulting Agreement by giving written notice to the Company three months prior to the proposed termination date, provided, however, Mr. Jones is required to provide an additional three months of transition services to the Company upon reasonable request by the Company. The Amended and Restated Consulting Agreement specifies monthly base retainer compensation of $21,666 per month until April 30, 2017; $15,000 per month from May 1, 2017 until April 30, 2018; $12,500 per month from May 1, 2018 until April 30, 2019; and $10,000 per month thereafter. Mr. Jones is also eligible to receive a cash bonus based on the achievement of certain performance metrics with a target of 35% of his base retainer for any given fiscal year. Such bonus is eligible to be increased to up to 150% of the target bonus in any fiscal year in which he meets certain performance thresholds established by the CEO of the Company and approved by the Board of Directors.
Contractual Obligations
The following table summarizes our significant contractual obligations as of December 31, 2017 ($ in thousands):
|
|
|
Total |
|
|
2018 |
|
|
2019 to 2020 |
|
|
2021 to 2022 |
|
|
After 2022 |
|
|||||
|
Purchase obligations |
|
$ |
2,158 |
|
|
$ |
942 |
|
|
$ |
1,216 |
|
|
$ |
- |
|
|
$ |
- |
|
|
Capital lease obligations |
|
|
11,209 |
|
|
|
5,461 |
|
|
|
5,635 |
|
|
|
113 |
|
|
|
- |
|
|
Operating lease obligations |
|
|
9,851 |
|
|
|
3,473 |
|
|
|
5,291 |
|
|
|
1,087 |
|
|
|
- |
|
|
Principal payments on long term debt (1) |
|
|
96,650 |
|
|
|
3,750 |
|
|
|
11,250 |
|
|
|
81,650 |
|
|
|
- |
|
|
Interest on swap agreement (2) |
|
|
1,590 |
|
|
|
795 |
|
|
|
795 |
|
|
|
- |
|
|
|
- |
|
|
Interest on Term Loan Facility (3) |
|
|
8,194 |
|
|
|
2,320 |
|
|
|
4,130 |
|
|
|
1,744 |
|
|
|
- |
|
|
Interest on Revolving Facility (4) |
|
|
5,608 |
|
|
|
1,288 |
|
|
|
2,576 |
|
|
|
1,744 |
|
|
|
- |
|
|
Total contractual obligations |
|
$ |
135,260 |
|
|
$ |
18,029 |
|
|
$ |
30,893 |
|
|
$ |
86,338 |
|
|
$ |
- |
|
|
(1) |
Amounts represent required principal debt payments on our Term Loan Facility and Revolving Facility. For a full description of the terms of our indebtedness and the related debt service requirements, see Note F. |
|
(2) |
Amounts represent fixed interest owed on the swap agreement. For further details of the swap agreement, see Note G. |
|
(3) |
Amounts represent interest payments due on the Term Loan Facility assuming principal payments are made as specified in the loan agreement and estimated interest rates based on the rates in effect at December 31, 2017. |
|
(4) |
Amounts represent interest payments due on the Revolving Facility based on the December 31, 2017 principal balance and estimated interest rates based on the interest rates in effect at December 31, 2017. |
Capital Expenditures
We currently forecast capital expenditures in order to execute on our business plan. The amount and timing of such capital expenditures will be determined by the volume of business, but we currently estimate that we will need to purchase approximately $18 million to $20 million of additional capital equipment during the next year. We plan to fund these expenditures with capital lease financing arrangements and cash. If we are unable to obtain such funding, we will need to make advances on our revolving credit facility in order to pay cash for these items.
74
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Recently Adopted and Issued Accounting Guidance
Adopted
In January 2017, the FASB issued ASU No. 2017-01, Business Combinations. This standard clarifies the definition of a business and provides guidance on when transactions should be accounted for as acquisitions of assets and when they should be accounted for as acquisitions of businesses. The Company early adopted this standard on July 1, 2017 and applied this guidance to the customer list that was acquired on August 1, 2017. The customer list acquired was not determined to meet the definition of a business under this standard and was therefore determined to be an asset acquisition.
In March 2016, the FASB issued Accounting Standards Update (“ASU”) No. 2016-09, Improvements to Employee Share-Based Payment Accounting. The standard update required excess tax benefits and tax deficiencies to be recorded directly through earnings as a component of income tax expense. Under previous GAAP, these differences were generally recorded in additional paid-in capital and thus had no impact on net income. The change impacted the computation of diluted earnings per share, and the cash flows associated with those items are now classified as operating activities on the condensed statements of consolidated cash flows. Entities were permitted to make an accounting policy election for the impact of forfeitures on the recognition of expense for share-based payment awards. Forfeitures could be estimated, as required under previous GAAP, or recognized when they occur.
The Company adopted this ASU on January 1, 2017 using the transition method prescribed for each applicable provision:
|
|
• |
Based on the implementation guidance, previously unrecognized excess tax benefits should be on a modified retrospective basis beginning in the period the guidance is adopted. Accordingly, the Company recorded an increase in deferred tax assets and an offsetting cumulative-effect adjustment to retained earnings of $6.4 million as of January 1, 2017 for excess tax benefits not previously recognized. |
|
|
• |
Based on the implementation guidance, all excess tax benefits and tax deficiencies related to share based compensation will be reported in net income (loss) on a prospective basis. For the year ended December 31, 2017, $0 in income (loss) was reported. |
|
|
• |
The Company has elected to retrospectively adopt the requirement to present cash flows related to excess tax benefits as cash flows from operating activities. This adoption had no effect on cash flows for the year ended December 31, 2017. |
|
|
• |
The Company has elected to recognize forfeitures in compensation cost as they occur. |
Issued
In August 2017 the FASB issued ASU 2017-12, Derivatives and Hedging. This standard refines hedge accounting to better align an entity’s risk management activities and financial reporting for hedging relationships through changes to both the designation and measurement guidance for qualifying hedging relationships and the presentation of hedge results. This update is effective for annual periods beginning after December 15, 2018 and interim periods within those annual periods. Early adoption is permitted. The Company does not expect the adoption of ASU 2017-12 to have a material effect on its consolidated financial statements.
In May 2017, the FASB issued ASU 2017-09, Compensation – Stock Compensation. This standard provides guidance related to the scope of stock option modification accounting, to reduce diversity in practice and reduce cost and complexity regarding existing guidance. This update is effective for annual periods beginning after December 15, 2017. Early adoption is permitted. The Company does not expect the adoption of ASU 2017-09 to have a material effect on its consolidated financial statements.
In January 2017 the FASB issued ASU No. 2017-04, Intangibles – Goodwill and Other: Simplifying the Test for Goodwill Impairment. This standard eliminates Step 2 of the goodwill impairment test. Instead, an entity should perform its annual or interim goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An entity should recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill
75
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
allocated to that reporting unit. This update is effective for annual and interim periods beginning after December 15, 2019. Early adoption is permitted for interim or annual goodwill impairment tests performed after January 1, 2017. The Company does not expect the adoption of ASU 2017-04 to have a material effect on its consolidated financial statements.
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows – Classification of Certain Cash Receipts and Cash Payments. The update clarifies how specific cash receipts and cash payments are classified and presented in the statement of cash flows. This update is effective for fiscal years and interim periods within those fiscal years beginning after December 15, 2017. Early adoption is permitted. The Company does not expect the adoption of ASU 2016-15 have a material effect on its consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases. The update was issued to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities, including for operating leases, on the balance sheet and disclosing key information about leasing arrangements. ASU 2016-02 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. The adoption of this ASU will result in an increase on the balance sheet for lease liabilities and right to use assets. The Company is currently evaluating the quantitative impact that adopting ASU 2016-02 will have on its consolidated financial statements and assessing any changes to its processes and controls.
In May 2014, the FASB issued ASU 2014-09, which amends FASB Accounting Standards Codification by creating Topic 606, Revenues from Contracts with Customers. This standard update calls for a number of revisions in the revenue recognition rules. In August 2015, the FASB deferred the effective date of this ASU to the first quarter of 2018, with early adoption permitted beginning in the first quarter of 2017. The ASU can be applied using a full retrospective method or a modified retrospective method of adoption. The Company has adopted this ASU on January 1, 2018 using a full retrospective method of adoption. Under this method, the Company will restate its results for each prior reporting period presented as if ASC 606 had been effective for those periods.
The adoption of this standard will require us to implement new revenue policies, procedures and internal controls related to revenue recognition. In addition, the adoption will result in enhanced financial statement disclosures surrounding the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The new standard impacts each of our two reportable segments differently due to the transactional nature of the Clinical Services Division versus the generally long-term nature of our Pharma Services Division contracts. The specific effect on our reportable segments is explained below:
Clinical Testing Revenue
Under the new standard, substantially all of our bad debt expense, which has historically been presented as part of general and administrative expense, is considered an implicit price concession and will be reported as a reduction in revenue. As a result of the new standard, there will be a material cumulative reduction in clinical revenue from previously reported periods and a similar reduction in general and administrative expenses.
Pharma Testing Revenue
The adoption of the new standard may result in changes to the timing of revenue recognition related to Pharma Services contracts as individual deliverables, for which revenue was previously recognized in the period when the deliverables were completed and invoiced, will be recognized over the remaining performance period under the new standard. Additionally, certain costs to obtain contracts, primarily for sales commissions, will be capitalized when incurred and will be amortized over the term of the contract. Under ASC 606, the Company is required to make estimates of the net sales price, including estimates of variable consideration, and recognize the estimated amount as revenue when it transfers control of the product or performance obligations to its customers. The estimation of variable consideration and the application of the related constraint, was not required under previous GAAP, variable consideration must now be determined using either an expected value or most likely amount method which requires the use of significant management judgment and estimates. The cumulative effect of this standard is not expected to result in a material change to our Pharma Services revenue.
76
NEOGENOMICS, INC.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (CONTINUED)
Off-Balance Sheet Arrangements
We do not use special purpose entities or other off-balance sheet financing techniques that we believe have, or are reasonably likely to have, a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity or capital resources.
Effects of Inflation
We do not believe that inflation has had a material impact on our business, revenues, or operating results during the periods presented.
77
NEOGENOMICS, INC.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Market risk is the potential loss arising from adverse changes in market rates and prices, such as foreign currency exchange rates, interest rates and other relevant market rate or price changes. We are exposed to market risks, including changes in interest rates and changes in foreign currency exchange rates.
Interest Rate Risk
The Company is exposed to market risk associated with changes in the LIBOR interest rate. The Company regularly evaluates its exposure to such changes and may elect to minimize this risk through the use of interest rate swap agreements. During the fourth quarter of 2016, the Company entered into a Credit Agreement which provides for a $75.0 million Term Loan Facility as well as a $75.0 million Revolving Credit Facility. Borrowings under these facilities bear interest at a variable rate based on one-month LIBOR plus a margin. To reduce the risk associated with changes in this variable rate, the Company has entered into an interest rate swap agreement with a notional amount of $50 million. As of December 31, 2017, the Company had approximately $46.7 million of unhedged variable rate debt under the senior secured credit facility. For further details regarding our significant accounting policies relating to derivative instruments and hedging activities, see Note B to our Consolidated Financial Statements included in this Annual Report.
Each quarter-point increase or decrease in the one-month LIBOR rate would result in a change in the Company's interest expense by approximately $116 thousand per year based on the unhedged debt outstanding at December 31, 2017.
Foreign Currency Exchange Risk
In 2017, we expanded into Europe and now transact business internationally. Our international revenues and expenses denominated in foreign currencies (primarily Swiss Francs), expose us to the risk of fluctuations in foreign currency exchange rates against the U.S. dollar. We do not hedge foreign currency exchange risks and do not currently feel that these risks are significant.
78
NEOGENOMICS, INC.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
79
Report of Independent Registered Public Accounting Firm
Stockholders and the Board of Directors of NeoGenomics, Inc.
Fort Myers, Florida
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of NeoGenomics, Inc. (the "Company") as of December 31, 2017 and 2016, the related consolidated statements of operations, comprehensive loss, redeemable convertible preferred stock and stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2017, and the related notes (collectively referred to as the "financial statements"). We also have audited the Company’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control – Integrated Framework: (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2017 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control – Integrated Framework: (2013) issued by COSO.
Explanatory Paragraph - Change in Accounting Principle
As discussed in Note B to the financial statements, the Company has changed its method of accounting for excess tax benefits related to employee shared-based payments in 2017 due to the adoption of Financial Accounting Standards Board Accounting Standards Update Number 2016-09, Improvements to Employee Share-Based Payment Accounting.
Basis for Opinions
The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Management’s Report on Internal Control Over Financial Reporting.” Our responsibility is to express an opinion on the Company’s financial statements and an opinion on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the financial statements included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the
80
company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Crowe Horwath LLP
We have served as the Company's auditor since 2014.
Indianapolis, Indiana
March 13, 2018
81
NEOGENOMICS INC.
(In thousands, except share amounts)
|
|
|
As of December 31, |
|
|||||
|
|
|
2017 |
|
|
2016 |
|
||
|
ASSETS |
|
|
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
12,821 |
|
|
$ |
12,525 |
|
|
Accounts receivable (net of allowance for doubtful accounts of $13,700 and $13,699, respectively) |
|
|
60,427 |
|
|
|
55,512 |
|
|
Inventories |
|
|
7,474 |
|
|
|
6,253 |
|
|
Other current assets |
|
|
4,241 |
|
|
|
4,535 |
|
|
Total current assets |
|
|
84,963 |
|
|
|
78,825 |
|
|
Property and equipment (net of accumulated depreciation of $40,530 and $27,102, respectively) |
|
|
36,504 |
|
|
|
34,036 |
|
|
Intangible assets, net |
|
|
74,165 |
|
|
|
77,064 |
|
|
Goodwill |
|
|
147,019 |
|
|
|
147,019 |
|
|
Other assets |
|
|
689 |
|
|
|
174 |
|
|
Total assets |
|
$ |
343,340 |
|
|
$ |
337,118 |
|
|
LIABILITIES, REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
10,450 |
|
|
$ |
16,782 |
|
|
Accrued compensation |
|
|
9,482 |
|
|
|
8,351 |
|
|
Accrued expenses and other liabilities |
|
|
6,144 |
|
|
|
4,247 |
|
|
Short-term portion of car loans |
|
|
49 |
|
|
|
92 |
|
|
Short-term portion of capital leases |
|
|
5,190 |
|
|
|
4,891 |
|
|
Short-term portion of term loan |
|
|
3,750 |
|
|
|
3,750 |
|
|
Total current liabilities |
|
|
35,065 |
|
|
|
38,113 |
|
|
Long-term liabilities |
|
|
|
|
|
|
|
|
|
Long-term portion of car loans |
|
|
20 |
|
|
|
110 |
|
|
Long-term portion of capital leases |
|
|
5,283 |
|
|
|
5,378 |
|
|
Long-term portion of term loan, net |
|
|
66,616 |
|
|
|
70,149 |
|
|
Revolving credit facility, net |
|
|
24,516 |
|
|
|
21,799 |
|
|
Deferred income tax liability, net |
|
|
6,307 |
|
|
|
14,973 |
|
|
Total long-term liabilities |
|
|
102,742 |
|
|
|
112,409 |
|
|
Total liabilities |
|
|
137,807 |
|
|
|
150,522 |
|
|
Commitments and contingencies - see Note L |
|
|
|
|
|
|
|
|
|
Redeemable convertible preferred stock: |
|
|
|
|
|
|
|
|
|
Series A Redeemable Convertible Preferred Stock, $0.001 par value, (50,000,000 shares authorized; and 6,864,000 and 6,600,000 shares issued and outstanding, respectively) |
|
|
32,615 |
|
|
|
22,873 |
|
|
Stockholders' equity |
|
|
|
|
|
|
|
|
|
Common stock, $.001 par value, (250,000,000 shares authorized; 80,462,574 and 78,571,158 shares issued and outstanding, respectively) |
|
|
80 |
|
|
|
79 |
|
|
Additional paid-in capital |
|
|
230,030 |
|
|
|
216,104 |
|
|
Accumulated other comprehensive income |
|
|
274 |
|
|
|
— |
|
|
Accumulated deficit |
|
|
(57,466 |
) |
|
|
(52,460 |
) |
|
Total stockholders’ equity |
|
|
172,918 |
|
|
|
163,723 |
|
|
Total liabilities, redeemable convertible preferred stock and stockholders' equity |
|
$ |
343,340 |
|
|
$ |
337,118 |
|
See notes to consolidated financial statements.
82
NEOGENOMICS INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
|
|
|
For the years ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
NET REVENUE |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical Services |
|
$ |
231,748 |
|
|
$ |
222,015 |
|
|
$ |
98,595 |
|
|
Pharma Services |
|
|
26,863 |
|
|
|
22,068 |
|
|
|
1,207 |
|
|
Total Revenue |
|
|
258,611 |
|
|
|
244,083 |
|
|
|
99,802 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenue |
|
|
138,295 |
|
|
|
133,704 |
|
|
|
56,046 |
|
|
GROSS MARGIN |
|
|
120,316 |
|
|
|
110,379 |
|
|
|
43,756 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative |
|
|
88,755 |
|
|
|
75,782 |
|
|
|
33,631 |
|
|
Research and development |
|
|
3,636 |
|
|
|
4,649 |
|
|
|
4,198 |
|
|
Sales and marketing |
|
|
24,543 |
|
|
|
23,910 |
|
|
|
11,562 |
|
|
Loss on sale of Path Logic |
|
|
1,058 |
|
|
|
— |
|
|
|
— |
|
|
Impairment charges |
|
|
— |
|
|
|
3,464 |
|
|
|
— |
|
|
Total operating expenses |
|
|
117,992 |
|
|
|
107,805 |
|
|
|
49,391 |
|
|
INCOME (LOSS) FROM OPERATIONS |
|
|
2,324 |
|
|
|
2,574 |
|
|
|
(5,635 |
) |
|
Interest expense and debt termination fees, net |
|
|
5,540 |
|
|
|
9,998 |
|
|
|
854 |
|
|
Other expense (income) |
|
|
265 |
|
|
|
- |
|
|
|
(2,000 |
) |
|
(Loss) before taxes |
|
|
(3,481 |
) |
|
|
(7,424 |
) |
|
|
(4,489 |
) |
|
Income tax benefit |
|
|
2,635 |
|
|
|
1,701 |
|
|
|
1,954 |
|
|
NET(LOSS) |
|
|
(846 |
) |
|
|
(5,723 |
) |
|
|
(2,535 |
) |
|
Deemed dividends on preferred stock |
|
|
3,645 |
|
|
|
18,011 |
|
|
|
40 |
|
|
Amortization of preferred stock beneficial conversion feature |
|
|
6,902 |
|
|
|
6,663 |
|
|
|
82 |
|
|
NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS |
|
$ |
(11,393 |
) |
|
$ |
(30,397 |
) |
|
$ |
(2,657 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET (LOSS) PER SHARE ATTRIBUTABLE TO COMMON STOCKHOLDERS |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.14 |
) |
|
$ |
(0.39 |
) |
|
$ |
(0.04 |
) |
|
Diluted |
|
$ |
(0.14 |
) |
|
$ |
(0.39 |
) |
|
$ |
(0.04 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
|
79,426 |
|
|
|
77,542 |
|
|
|
60,526 |
|
|
Diluted |
|
|
79,426 |
|
|
|
77,542 |
|
|
|
60,526 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See notes to consolidated financial statements.
83
NEOGENOMICS INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
|
|
|
For the years ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
NET (LOSS) |
|
$ |
(846 |
) |
|
$ |
(5,723 |
) |
|
$ |
(2,535 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE INCOME, NET OF TAX: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustments |
|
44 |
|
|
|
— |
|
|
|
— |
|
|
|
Gain on effective cash flow hedge |
|
|
230 |
|
|
|
— |
|
|
|
— |
|
|
Total other comprehensive income, net of tax |
|
|
274 |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COMPREHENSIVE (LOSS) |
|
$ |
(572 |
) |
|
$ |
(5,723 |
) |
|
$ |
(2,535 |
) |
See notes to consolidated financial statements.
84
NEOGENOMICS INC.
CONSOLIDATED STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY
(In thousands, except share amounts)
|
|
|
Series A Redeemable Convertible Preferred Stock |
|
|
Common Stock |
|
|
Additional Paid-In |
|
|
Accumulated Other Comprehensive |
|
|
Accumulated |
|
|
|
|
|
|||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income |
|
|
Deficit |
|
|
Total |
|
||||||||
|
BALANCE, DECEMBER 31, 2014 |
|
|
— |
|
|
$ |
— |
|
|
|
60,242,818 |
|
|
$ |
60 |
|
|
$ |
79,751 |
|
|
$ |
— |
|
|
$ |
(19,406 |
) |
|
$ |
60,405 |
|
|
Common stock issuance ESPP plan |
|
|
— |
|
|
|
— |
|
|
|
73,958 |
|
|
|
— |
|
|
|
369 |
|
|
|
— |
|
|
|
— |
|
|
|
369 |
|
|
Issuance of Series A Preferred Stock |
|
|
14,666,667 |
|
|
|
28,480 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Stock issuance fees and expenses |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(148 |
) |
|
|
— |
|
|
|
— |
|
|
|
(148 |
) |
|
Issuance of restricted stock |
|
|
— |
|
|
|
— |
|
|
|
11,440 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of common stock for stock options |
|
|
— |
|
|
|
— |
|
|
|
492,091 |
|
|
|
1 |
|
|
|
713 |
|
|
|
— |
|
|
|
— |
|
|
|
714 |
|
|
Tax benefit from stock option award activity |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
118 |
|
|
|
— |
|
|
|
— |
|
|
|
118 |
|
|
Issuance of common stock to fund acquisition |
|
|
— |
|
|
|
— |
|
|
|
15,000,000 |
|
|
|
15 |
|
|
|
102,495 |
|
|
|
— |
|
|
|
— |
|
|
|
102,510 |
|
|
Beneficial conversion feature |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
44,720 |
|
|
|
— |
|
|
|
— |
|
|
|
44,720 |
|
|
Deemed dividends on preferred stock |
|
|
— |
|
|
|
40 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(40 |
) |
|
|
(40 |
) |
|
Amortization of beneficial conversion feature |
|
|
— |
|
|
|
82 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(82 |
) |
|
|
(82 |
) |
|
Stock compensation expense - warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
590 |
|
|
|
— |
|
|
|
— |
|
|
|
590 |
|
|
Stock comp. exp. - options and restricted stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,889 |
|
|
|
— |
|
|
|
— |
|
|
|
2,889 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2,535 |
) |
|
|
(2,535 |
) |
|
BALANCE, DECEMBER 31, 2015 |
|
|
14,666,667 |
|
|
$ |
28,602 |
|
|
|
75,820,307 |
|
|
$ |
76 |
|
|
$ |
231,497 |
|
|
$ |
— |
|
|
$ |
(22,063 |
) |
|
$ |
209,510 |
|
|
Common stock issuance ESPP plan |
|
|
— |
|
|
|
— |
|
|
|
98,672 |
|
|
|
— |
|
|
|
736 |
|
|
|
— |
|
|
|
— |
|
|
|
736 |
|
|
Redemption of Series A Preferred Stock |
|
|
(8,066,667 |
) |
|
|
(55,000 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Stock issuance fees and expenses |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(267 |
) |
|
|
— |
|
|
|
— |
|
|
|
(267 |
) |
|
Issuance of restricted stock |
|
|
— |
|
|
|
— |
|
|
|
43,332 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of stock for warrant exercise |
|
|
— |
|
|
|
— |
|
|
|
165,375 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of common stock for stock options |
|
|
— |
|
|
|
— |
|
|
|
2,443,472 |
|
|
|
3 |
|
|
|
3,296 |
|
|
|
— |
|
|
|
— |
|
|
|
3,299 |
|
|
Beneficial conversion feature reversal |
|
|
— |
|
|
|
24,596 |
|
|
|
— |
|
|
|
— |
|
|
|
(24,596 |
) |
|
|
— |
|
|
|
— |
|
|
|
(24,596 |
) |
|
Deemed dividends on preferred stock |
|
|
— |
|
|
|
18,011 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(18,011 |
) |
|
|
(18,011 |
) |
|
Change in beneficial conversion feature |
|
|
— |
|
|
|
6,663 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(6,663 |
) |
|
|
(6,663 |
) |
|
Stock compensation expense - warrants |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
460 |
|
|
|
— |
|
|
|
— |
|
|
|
460 |
|
|
Stock compensation expense - options and restricted stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,978 |
|
|
|
— |
|
|
|
— |
|
|
|
4,978 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(5,723 |
) |
|
|
(5,723 |
) |
|
BALANCE, DECEMBER 31, 2016 - As previously filed |
|
|
6,600,000 |
|
|
|
22,873 |
|
|
|
78,571,158 |
|
|
|
79 |
|
|
|
216,104 |
|
|
|
— |
|
|
|
(52,460 |
) |
|
|
163,723 |
|
|
Cumulative effect of change in accounting policy: |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Adjustment for adoption of ASU 2016-09 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
6,387 |
|
|
|
6,387 |
|
|
BALANCE, DECEMBER 31, 2016 - As adjusted |
|
|
6,600,000 |
|
|
|
22,873 |
|
|
|
78,571,158 |
|
|
|
79 |
|
|
|
216,104 |
|
|
|
— |
|
|
|
(46,073 |
) |
|
|
170,110 |
|
|
Common stock issuance ESPP plan |
|
|
— |
|
|
|
— |
|
|
|
108,599 |
|
|
|
— |
|
|
|
844 |
|
|
|
— |
|
|
|
— |
|
|
|
844 |
|
|
Issuance of Series A Preferred Stock |
|
|
264,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Stock issuance fees and expenses |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(218 |
) |
|
|
— |
|
|
|
— |
|
|
|
(218 |
) |
|
Foreign currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
44 |
|
|
|
— |
|
|
|
44 |
|
|
Gain on effective cash flow hedge |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
230 |
|
|
|
— |
|
|
|
230 |
|
|
Issuance of restricted stock |
|
|
— |
|
|
|
— |
|
|
|
822,711 |
|
|
|
1 |
|
|
|
4,094 |
|
|
|
— |
|
|
|
— |
|
|
|
4,095 |
|
|
Issuance of stock for warrant exercise |
|
|
— |
|
|
|
— |
|
|
|
364,600 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
85
NEOGENOMICS INC.
|
|
Series A Redeemable Convertible Preferred Stock |
|
|
Common Stock |
|
|
Additional Paid-In |
|
|
Accumulated Other Comprehensive |
|
|
Accumulated |
|
|
|
|
|
||||||||||||||
|
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income |
|
|
Deficit |
|
|
Total |
|
||||||||
|
Issuance of common stock for stock options |
|
|
— |
|
|
|
— |
|
|
|
595,506 |
|
|
|
— |
|
|
|
1,960 |
|
|
|
— |
|
|
|
— |
|
|
|
1,960 |
|
|
Deemed dividends on preferred stock |
|
|
— |
|
|
|
3,645 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,645 |
) |
|
|
(3,645 |
) |
|
Amortization of beneficial conversion feature |
|
|
— |
|
|
|
6,097 |
|
|
|
— |
|
|
|
— |
|
|
|
805 |
|
|
|
— |
|
|
|
(6,902 |
) |
|
|
(6,097 |
) |
|
ESPP Expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
96 |
|
|
|
— |
|
|
|
— |
|
|
|
96 |
|
|
Stock compensation expense - options and restricted stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
6,345 |
|
|
|
— |
|
|
|
— |
|
|
|
6,345 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(846 |
) |
|
|
(846 |
) |
|
BALANCE, DECEMBER 31, 2017 |
|
|
6,864,000 |
|
|
|
32,615 |
|
|
|
80,462,574 |
|
|
|
80 |
|
|
|
230,030 |
|
|
|
274 |
|
|
|
(57,466 |
) |
|
|
172,918 |
|
See notes to consolidated financial statements.
86
NEOGENOMICS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
|
|
For the years ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
CASH FLOWS FROM OPERATING ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (Loss) |
|
$ |
(846 |
) |
|
$ |
(5,723 |
) |
|
$ |
(2,535 |
) |
|
Adjustments to reconcile net (loss) to net cash provided by operating activities, net of business acquisition: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Impact of tax valuation allowance |
|
|
— |
|
|
|
— |
|
|
|
(2,066 |
) |
|
Depreciation |
|
|
15,596 |
|
|
|
15,937 |
|
|
|
6,730 |
|
|
Impairment/loss on sale of assets |
|
|
253 |
|
|
|
3,464 |
|
|
|
— |
|
|
Loss on sale of business |
|
|
1,058 |
|
|
|
— |
|
|
|
— |
|
|
Amortization of intangibles |
|
|
6,995 |
|
|
|
7,272 |
|
|
|
412 |
|
|
Loss on extinguishment of debt |
|
|
— |
|
|
|
1,099 |
|
|
|
— |
|
|
Amortization of debt issue costs |
|
|
440 |
|
|
|
3,497 |
|
|
|
— |
|
|
Stock based compensation |
|
|
6,441 |
|
|
|
5,438 |
|
|
|
3,479 |
|
|
Provision for bad debts |
|
|
18,649 |
|
|
|
11,856 |
|
|
|
2,318 |
|
|
Changes in assets and liabilities, net of business acquisition: |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Increase) in accounts receivable, net of write-offs |
|
|
(24,243 |
) |
|
|
(18,425 |
) |
|
|
(3,215 |
) |
|
(Increase) in inventories |
|
|
(1,423 |
) |
|
|
(1,145 |
) |
|
|
(896 |
) |
|
(Increase) decrease in other assets |
|
|
(29 |
) |
|
|
(44 |
) |
|
|
11 |
|
|
(Increase) decrease in other current assets |
|
|
(310 |
) |
|
|
354 |
|
|
|
(3,748 |
) |
|
Increase (decrease) in accounts payable and other liabilities |
|
|
(4,544 |
) |
|
|
(2,103 |
) |
|
|
5,903 |
|
|
Net cash provided by operating activities |
|
|
18,037 |
|
|
|
21,477 |
|
|
|
6,393 |
|
|
CASH FLOWS FROM INVESTING ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
Acquisition, net of cash acquired of $0, $0 and $890 |
|
|
— |
|
|
|
1,035 |
|
|
|
(72,940 |
) |
|
Purchases of property and equipment |
|
|
(13,690 |
) |
|
|
(7,536 |
) |
|
|
(2,215 |
) |
|
Net cash used in investing activities |
|
|
(13,690 |
) |
|
|
(6,501 |
) |
|
|
(75,155 |
) |
|
CASH FLOWS FROM FINANCING ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
Advances from revolving credit facility |
|
|
2,496 |
|
|
|
12,856 |
|
|
|
10,002 |
|
|
Repayment of capital lease obligations |
|
|
(5,424 |
) |
|
|
(5,293 |
) |
|
|
(4,115 |
) |
|
Proceeds from term loan |
|
|
— |
|
|
|
75,000 |
|
|
|
55,022 |
|
|
Redemption of preferred stock |
|
|
— |
|
|
|
(55,000 |
) |
|
|
— |
|
|
Repayment of term loan |
|
|
(3,753 |
) |
|
|
(55,000 |
) |
|
|
— |
|
|
Payments of debt issue costs |
|
|
— |
|
|
|
(2,202 |
) |
|
|
(3,351 |
) |
|
Issuance of common stock for the exercise of options, warrants and ESPP shares, net of transaction expenses |
|
|
2,586 |
|
|
|
3,768 |
|
|
|
935 |
|
|
Net cash (used in) provided by financing activities |
|
|
(4,095 |
) |
|
|
(25,871 |
) |
|
|
58,493 |
|
|
Effects of foreign exchange rate changes on cash and cash equivalents |
|
|
44 |
|
|
|
— |
|
|
|
— |
|
|
Net change in cash and cash equivalents |
|
|
296 |
|
|
|
(10,895 |
) |
|
|
(10,269 |
) |
|
Cash and cash equivalent, beginning of year |
|
|
12,525 |
|
|
|
23,420 |
|
|
|
33,689 |
|
|
Cash and cash equivalents, end of year |
|
$ |
12,821 |
|
|
$ |
12,525 |
|
|
$ |
23,420 |
|
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid |
|
$ |
5,155 |
|
|
$ |
5,423 |
|
|
$ |
911 |
|
|
Income taxes paid |
|
|
284 |
|
|
|
290 |
|
|
|
25 |
|
|
Supplemental disclosure of non-cash investing and financing information: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Equipment acquired under capital lease/loan obligations |
|
|
5,728 |
|
|
|
6,057 |
|
|
|
4,813 |
|
|
Fair value of common stock issued to fund acquisition |
|
|
- |
|
|
|
- |
|
|
|
102,510 |
|
|
Fair value of preferred stock issued to fund acquisition |
|
|
- |
|
|
|
- |
|
|
|
73,200 |
|
|
Fair value of restricted stock issued to fund purchase of customer list |
|
|
4,095 |
|
|
|
- |
|
|
|
- |
|
See notes to consolidated financial statements
87
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
Note A – Nature of Business and Basis of Presentation
NeoGenomics, Inc., a Nevada corporation (the “Parent” or the “Parent Company”), and its subsidiaries, NeoGenomics Laboratories, Inc., a Florida corporation (“NeoGenomics Laboratories”), Path Labs LLC., a Delaware Limited Liability Corporation (“Path Logic”) which was sold on August 1, 2017, Clarient Inc. and its wholly-owned subsidiary Clarient Diagnostic Services, Inc. (“Clarient”), NeoGenomics Bioinformatics, Inc. and NeoGenomics Europe, SA (collectively referred to as “we”, “us”, “our”, “NeoGenomics”, or the “Company”), operates as a certified “high complexity” clinical laboratory in accordance with the federal government’s Clinical Laboratory Improvement Act, as amended (“CLIA”), and is dedicated to the delivery of clinical diagnostic services to pathologists, oncologists, urologists, hospitals, and other laboratories as well as providing clinical trial services to pharmaceutical firms.
The accompanying consolidated financial statements include the accounts of the Parent and all Subsidiaries. Significant intercompany accounts and balances have been eliminated in consolidation.
Segment Reporting
The Company reports its activities in two operating segments, the Clinical Services Segment and the Pharma Services Segment. These reportable segments deliver testing services to hospitals, pathologists, oncologists, clinicians, pharmaceutical firms and researchers and represent 100% of the Company’s consolidated assets, net revenues and net income for each of the three years ended December 31, 2017, 2016 and 2015, respectively. For further financial information about these segments, see Note Q to our consolidated financial statements included in this Annual Report.
Note B – Summary of Significant Accounting Policies
Use of Estimates
The Company prepares its consolidated financial statements in conformity with accounting principles generally accepted in the United States of America. These principles require management to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, together with amounts disclosed in the related notes to the consolidated financial statements. Actual results and outcomes may differ from management’s estimates, judgments and assumptions. Significant estimates, judgments and assumptions used in these consolidated financial statements include, but are not limited to, those related to revenues, accounts receivable and related allowances, contingencies, useful lives and recovery of long-term assets and intangible assets, income taxes and valuation allowances, stock-based compensation and impairment analysis of goodwill. These estimates, judgments, and assumptions are reviewed periodically and the effects of material revisions in estimates are reflected in the consolidated financial statements prospectively from the date of the change in estimate.
Revenue Recognition
Clinical Services
The Company recognizes revenues when (a) the price is fixed or determinable, (b) persuasive evidence of an arrangement exists, (c) the service is performed and (d) collectability of the resulting receivable is reasonably assured. The Company’s specialized diagnostic services are performed based on a written test requisition form or electronic equivalent and revenues are recognized once the diagnostic services have been performed, and the results have been delivered to the ordering physician. These diagnostic services are billed to various payers, including Medicare, commercial insurance companies, other directly billed healthcare institutions such as hospitals and clinics, and individuals. The Company reports revenues from contracted payers, including Medicare, certain insurance companies and certain healthcare institutions, based on the contractual rate, or in the case of Medicare, published fee schedules. The Company reports revenues from non-contracted payers, including certain insurance companies and individuals, based on the amount expected to be collected. The difference between the amount billed and the amount estimated to be collected from non-contracted payers is recorded as a contractual allowance to arrive at the reported net revenues. The expected revenues from non-contracted payers are based on the historical
88
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
collection experience of each payer or payer group, as appropriate. The Company records revenues from patient pay tests net of a large discount and as a result recognizes minimal revenue on those tests. The Company regularly reviews its historical collection experience for non-contracted payers and adjusts its expected revenues for current and subsequent periods accordingly.
Pharma Services
The Company’s Pharma Services Division generally enters into contracts with pharmaceutical and biotech customers as well as other CROs to provide Research and Clinical Trial services ranging in duration from one month to several years. The contract terms generally provide for payments based on a unit-of-service arrangement. Revenue on these arrangements is recognized when there is persuasive evidence of an arrangement, the service offering has been delivered to the customer, the arrangement consideration is determinable and the collection of the fees is reasonably assured. The Company recognizes revenue in the period in which the unit is completed. Service unit elements largely consist of analytical testing services and related project support activities.
Most contracts are terminable by the customer, either immediately or according to advance notice terms specified within the contracts. All contracts require payment of fees to the Company for services rendered through the date of termination and may require payment for subsequent services necessary to conclude the study or close out the contract. Final settlement amounts are agreed upon with the customer and included in Service revenue when realization is reasonably assured.
The table below shows the adjustments made to gross service revenue to arrive at net revenues, the amount reported on our statement of operations (in thousands):
|
|
|
For the Years ended December 31, |
|
|||||||||
|
|
|
2017 (1) |
|
|
2016 |
|
|
2015 |
|
|||
|
Gross service revenues |
|
$ |
360,174 |
|
|
$ |
493,678 |
|
|
$ |
225,057 |
|
|
Total contractual adjustments and discounts |
|
|
(101,563 |
) |
|
|
(249,595 |
) |
|
|
(125,255 |
) |
|
Net service revenues |
|
$ |
258,611 |
|
|
$ |
244,083 |
|
|
$ |
99,802 |
|
(1) In 2017, NeoGenomics lowered its’ patient fee schedule; which led to the reduction in gross service revenues. The fee schedule reduction had a minimal impact on net service revenues.
Cost of Revenue
Cost of revenue includes payroll and payroll related costs for performing tests, depreciation of laboratory equipment, rent for laboratory facilities, laboratory reagents, probes and supplies, and delivery and courier costs relating to the transportation of specimens to be tested.
Shipping Costs
The Company has a significant expense related to shipping specimens to our facility for testing and this cost is for contract couriers, commercial airline flights and charges from FedEx to ship specimens to our facility. We also incur expenses returning samples and slides to our clients. We had approximately $10.8 million, $10.3 million and $3.6 million in outsourced shipping expenses for the years ended December 31, 2017, 2016 and 2015, respectively, and these costs were included in our cost of revenue.
Advertising Costs
Advertising costs are expensed at the time they are incurred and are not material for the years ended December 31, 2017, 2016 and 2015.
89
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
Research and development (“R&D”) costs are expensed as incurred. R&D expenses consist of cash and equity compensation and benefits for R&D personnel, amortization of intangibles, supplies, inventory and payment for samples to complete validation studies. These expenses are incurred to develop new genetic tests.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are comprised of amounts due from sales of the Company’s specialized diagnostic services and are recorded at the billed amount, net of discounts and contractual allowances. The allowance for doubtful accounts is estimated based on the aging of accounts receivable with each payer category and the historical data on bad debts in these aging categories. In addition, the allowance is adjusted periodically for other relevant factors, including regularly assessing the state of our billing operations in order to identify issues which may impact the collectability of receivables or allowance estimates. Revisions to the allowance are recorded as an adjustment to bad debt expense within general and administrative expenses. After appropriate collection efforts have been exhausted, specific receivables deemed to be uncollectible are charged against the allowance in the period they are deemed uncollectible. Recoveries of receivables previously written-off are recorded as credits to the allowance. Our estimates of net revenue are subject to change based on the contractual status and payment policies of the third party payers with whom we deal. We regularly refine our estimates in order to make our estimated revenue as accurate as possible based on our most recent collection experience with each third party payer.
Changes in the allowance for doubtful accounts are as follows (in thousands):
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Beginning balance – allowance for doubtful accounts |
|
$ |
13,699 |
|
|
$ |
4,759 |
|
|
$ |
4,180 |
|
|
Provision for doubtful accounts |
|
|
18,649 |
|
|
|
11,856 |
|
|
|
2,318 |
|
|
Write-offs |
|
|
(18,648 |
) |
|
|
(2,916 |
) |
|
|
(1,739 |
) |
|
Ending balance – allowance for doubtful accounts |
|
$ |
13,700 |
|
|
$ |
13,699 |
|
|
$ |
4,759 |
|
Foreign Currency
The functional currency for our subsidiary outside of the U.S. is the applicable local currency. We translate the financial statements of the subsidiary into U.S. dollars using average monthly exchange rates. Translation gains and losses are recorded in accumulated other comprehensive income (“AOCI”) as a component of stockholders' equity.
Statements of Cash Flows
For purposes of the consolidated statements of cash flows, we consider all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents.
Fair Value of Financial Instruments
The carrying value of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and other liabilities, and other current assets and liabilities, including our revolving credit facility are considered reasonable estimates of their respective fair values due to their short-term nature. The Company maintains its cash and cash equivalents with domestic financial institutions that the Company believes to be of high credit standing. The Company believes that, as of December 31, 2017, its concentration of credit risk related to cash and cash equivalents was not significant. The carrying value of the Company’s long-term capital lease obligations and term debt approximates its fair value based on the current market conditions for similar instruments. The Company entered into an interest rate swap agreement in December of 2016, see Note G- Derivative Instruments and Hedging Activities. At December 31, 2017, the fair value of the derivative financial instrument was $352,000, which was included in the balance sheet as other assets and reflected in AOCI. At December 31, 2016, the fair value of the derivative financial instrument was not considered to be significant and therefore, was not recorded on the balance sheet nor was the change in value reflected through AOCI.
90
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. A fair value hierarchy has been established based on three levels of inputs, of which the first two are considered observable and the last unobservable.
Level 1: Quoted prices in active markets for identical assets or liabilities. These are typically obtained from real-time quotes for transactions in active exchange markets involving identical assets.
Level 2: Inputs, other than quoted prices included within Level 1, which are observable for the asset or liability, either directly or indirectly. These are typically obtained from readily-available pricing sources for comparable instruments.
Level 3: Unobservable inputs, where there is little or no market activity for the asset or liability. These inputs reflect the reporting entity’s own assumptions of the data that market participants would use in pricing the asset or liability, based on the best information available in the circumstances.
Concentrations of Credit Risk
Concentrations of credit risk with respect to revenue and accounts receivable are primarily limited to certain clients and geographies to which the Company provides a significant volume of its services, and to specific payers of our services such as Medicare and individual insurance companies. The Company’s client base consists of a large number of geographically dispersed clients diversified across various customer types. For the years ended December 31, 2017, 2016 and 2015, no clients accounted for more than 5% of revenue. Due to the acquisition of Clarient, our concentration of revenue shifted from Florida to California. For the years ended December 31, 2017, 2016 and 2015, revenue derived from the State of California accounted for 21.1%, 24.0% and 20.2%, respectively, of total revenue. For the years ended December 31, 2017, 2016 and 2015, revenue derived from the State of Florida accounted for 13.9%, 15.0% and 20.5%, respectively, of total revenue.
Inventories
Inventories, which consist principally of testing supplies, are valued at the lower of cost or market, using the first-in, first-out method (FIFO).
Other Current Assets
As of December 31, 2017, 2016 and 2015, other current assets consist primarily of prepaid expenses relating to contracts for laboratory and computer equipment maintenance.
Property and Equipment
Property and equipment are recorded at cost, net of accumulated depreciation and amortization. Property and equipment generally includes purchases of items with a cost greater than $1,000 and a useful life greater than one year. Depreciation and amortization are computed on the straight-line basis over the estimated useful lives of the assets. Leasehold improvements and property and equipment under capital leases are amortized over the shorter of the related lease terms or their estimated useful lives. Costs incurred in connection with the development of internal-use software are capitalized in accordance with the accounting standard for internal-use software, and are amortized over the expected useful life of the software, generally 3-5 years. We perform a fair value assessment on property and equipment acquired in a business combination and record the fair value as the cost basis for those assets.
The Company periodically reviews the estimated useful lives of property and equipment. Changes to the estimated useful lives are recorded prospectively from the date of the change. Upon retirement or sale, the cost of the assets disposed of and the related accumulated depreciation are removed from the accounts and any resulting gain or loss is included in income (loss) from operations. Repairs and maintenance costs are expensed as incurred.
91
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
Intangible assets with finite useful lives are recorded at fair value or cost, less accumulated amortization. At December 31, 2017, we had two classes of assets, each class is amortized over its estimated service period using the straight-line method. We periodically review the estimated pattern in which the economic benefits will be consumed and adjust the amortization period and pattern to match our estimate.
At December 31, 2017, the Company’s intangible assets were related to customer relationships acquired through the acquisition of Clarient as well as customer relationships and a non-compete agreement related to the purchase of a customer list.
Goodwill
The Company evaluates goodwill on an annual basis in the fourth quarter or more frequently if management believes indicators of impairment exist. Such indicators could include, but are not limited to (1) a significant adverse change in legal factors or in business climate, (2) unanticipated competition, or (3) an adverse action or assessment by a regulator. The Company first assesses qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, including goodwill. If management concludes that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, management conducts a two-step quantitative goodwill impairment test. The first step of the impairment test involves comparing the fair value of the applicable reporting unit with its carrying value. The Company estimates the fair values of its reporting units using a combination of the income, or discounted cash flows, approach and the market approach, which utilizes comparable companies’ data. If the carrying amount of a reporting unit exceeds the reporting unit’s fair value, management performs the second step of the goodwill impairment test. The second step of the goodwill impairment test involves comparing the implied fair value of the affected reporting unit’s goodwill with the carrying value of that goodwill. The amount, by which the carrying value of the goodwill exceeds its implied fair value, if any, is recognized as an impairment loss. The Company’s evaluation of goodwill completed during the fourth quarter resulted in no impairment losses.
Recoverability and Impairment of Long-Lived Assets
The Company reviews the recoverability of its long-lived assets (property and equipment, and intangible assets) if events or changes in circumstances indicate the assets may be impaired. Evaluation of possible impairment is based on the Company’s ability to recover the asset from the expected future pretax cash flows (undiscounted and without interest charges) of the related operations. If the expected undiscounted pretax cash flows are less than the carrying amount of such asset, an impairment loss is recognized for the difference between the estimated fair value and carrying amount of the asset. No impairment losses were recognized in the years ended December 31, 2017 or 2015. The Company recognized approximately $3.5 million in impairment losses for the year ended December 31, 2016. See Note P for further details.
Debt Issuance Costs
We record debt issuance costs related to our debt liabilities as direct deductions from the carrying amount of the debt. The costs are amortized to interest expense over the life of the debt using the effective interest method.
Derivative Instruments and Hedging Activities
The Company uses derivative instruments to manage risks related to interest expense. We account for derivatives in accordance with Financial Accounting Standards Board (“FASB”) ASC Topic 815, which establishes accounting and reporting standards requiring that derivative instruments be recorded on the balance sheet as either an asset or liability and measured at fair value. Additionally, changes in the derivative's fair value will be recognized currently in earnings unless specific hedge accounting criteria are met.
92
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
In December of 2016, the Company entered into an interest rate swap agreement. The interest rate swap agreement effectively converts a portion of the Companies floating rate debt to a fixed rate, thereby reducing the impact on future changes in interest rates.
In accordance with ASC 815, the Company has designated the interest rate swap as a cash flow hedge. As the specific terms and notional amounts of the derivative financial instrument match those of the floating rate debt being hedged, the derivative instrument is assumed to be a perfectly effective hedge and, accordingly, there is no impact to the Company's consolidated statements of operations. At December 31, 2017, the fair value of the derivative financial instrument was $352,000, which was included in the balance sheet as other assets and reflected in AOCI. At December 31, 2016, it was determined that the fair value of this instrument was not significant and, therefore, is not recorded on the balance sheet as an asset/liability nor is the change in value reflected through AOCI. The instrument will be evaluated on a monthly basis and resulting increases/decreases will be recorded as a component of AOCI and reclassified to the consolidated statement of operations as interest is paid. Cash flows from the interest rate swap are to be included in operating activities on the consolidated statement of cash flows.
For further information on derivative instruments and hedging activities, see Note G to our Consolidated Financial Statements included in this Annual Report on Form 10-K.
Series A Redeemable Convertible Preferred Stock
The Company has classified the Series A Redeemable Convertible Preferred Stock (“Series A Preferred Stock”) as temporary equity on the consolidated balance sheet due to certain deemed liquidation events that are outside the Company’s control. These events include the following:
|
|
• |
Acquisition of 50% or more of the voting securities of the Company; |
|
|
• |
Consolidation, merger or corporate reorganization in which the stockholders of the Company immediately prior to such consolidation, merger or reorganization own less than 50% of the voting power immediately after the consolidation, merger or reorganization; |
|
|
• |
Sale, lease, license, transferor disposition of all or substantially all of the assets, technology or intellectual property of the Company. |
We evaluated our Series A Preferred Stock upon issuance in order to determine classification as to permanent or temporary equity and whether or not the instrument contains an embedded derivative that requires bifurcation. This analysis followed the whole instrument approach which compares an individual feature against the entire instrument which includes that feature. This analysis was based on a consideration of the economic characteristics and risk of the Series A Preferred Stock.
We evaluated all of the stated and implied substantive terms and features, including: (i) redemption (Purchase Call Option) on the Series A Preferred Stock allowing the Company to redeem the Series A Preferred Stock at any time, (ii) required redemption contingent if we raise capital, (iii) required redemption in the event of certain deemed liquidation events (in essence, any change in control of the Company), (iv) conversion (Written Call Option) on the underlying shares if after three years the stock trades at $8.00 for thirty trading days, and (v) conversion (Contingent Forward) on the underlying shares automatically at the ten year anniversary of the issue date.
As a result of this analysis, we concluded that the Series A Preferred Stock represented an equity host and, therefore, the redemption feature of the Series A Preferred Stock was not considered to be clearly and closely related to the associated equity host instrument. However, the redemption features did not meet the net settlement criteria of a derivative and, therefore, were not considered embedded derivatives that required bifurcation.
We also concluded that the conversion rights under the Series A Preferred Stock were clearly and closely related to the equity host instrument. Accordingly, the conversion rights features on the Series A Preferred Stock were not considered an embedded derivative that required bifurcation.
93
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The issuance of the Company's Series A Preferred Stock generated a beneficial conversion feature, which arises when a debt or equity security is issued with an embedded conversion option that is beneficial to the investor or in the money at inception because the conversion option has an effective strike price that is less than the market price of the underlying stock at the commitment date. We recognized this beneficial conversion feature by allocating the intrinsic value of the conversion option, which is the number of shares of common stock available upon conversion multiplied by the difference between the effective conversion price per share and the fair value of common stock per share on the commitment date, to additional paid-in capital, resulting in a discount on the Series A Preferred Stock. NeoGenomics is accreting the discount from the date of issuance through the earliest conversion date, which is three years. Accretion expense is recognized as dividend equivalents over the three year period. Upon redemption of any shares of preferred stock by the Company prior to any beneficial conversion feature being realized by the holder, the amount of beneficial conversion related to the number of shares redeemed that was accreted as dividends would be reversed, and the entire amount of beneficial conversion feature recorded in accumulated additional paid-in-capital would be reversed.
Income Taxes
We compute income taxes in accordance with ASC Topic 740, Income Taxes. Under ASC Topic 740, deferred taxes are recognized for the tax consequences of temporary differences by applying enacted statutory rates applicable to future years to differences between the financial statement carrying amounts and the tax bases of existing assets and liabilities. Also, the effect on deferred taxes of a change in tax rates is recognized in income in the period that included the enactment date. Temporary differences between financial and tax reporting arise primarily from the use of different depreciation methods and lives for property and equipment and recognition of bad debts and various other expenses that have been allowed for or accrued for financial statement purposes but are not currently deductible for income tax purposes.
The provision for income taxes, including the effective tax rate and analysis of potential tax exposure items, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowances deemed necessary to recognize deferred tax assets at an amount that is more likely than not to be realized. We evaluate tax positions that have been taken or are expected to be taken in our tax returns, and record a liability for uncertain tax positions, if deemed necessary. We follow a two-step approach to recognizing and measuring uncertain tax positions. First, tax positions are recognized if the weight of available evidence indicates that it is more likely than not that the position will be sustained upon examination, including resolution of related appeals or litigation processes, if any. Second, the tax position is measured as the largest amount of tax benefit that has a greater than 50% likelihood of being realized upon settlement. We recognize interest and penalties related to unrecognized tax benefits in the provision for income taxes in the accompanying consolidated financial statements. During the years ended December 31, 2017, 2016 and 2015, we do not believe we had any significant uncertain tax positions, nor did we have any provision for interest or penalties related to such positions.
Stock-Based Compensation
We measure compensation expense for stock-based awards to employees, non-employee contracted physicians, and directors based upon the awards’ initial grant-date fair value. The estimated grant-date fair value of the award is recognized as expense over the requisite service period using the straight-line method. The fair value of awards to non-employees are then marked-to-market each reporting period until vesting criteria are met.
We estimate the fair value of stock options and warrants using a trinomial lattice model. This model is affected by our stock price on the date of the grant as well as assumptions regarding a number of highly complex and subjective variables. These variables include the expected term of the option, expected risk-free rates of return, the expected volatility of our common stock, and expected dividend yield, each of which is more fully described below. The assumptions for expected term and expected volatility are the two assumptions that significantly affect the grant date fair value.
94
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
Expected Term: The expected term of an option is the period of time that the option is expected to be outstanding. The average expected term is determined using a trinomial lattice simulation model.
Risk-free Interest Rate: We base the risk-free interest rate used in the trinomial lattice valuation method on the implied yield at the grant date of the U.S. Treasury zero-coupon issue with an equivalent term to the stock-based award being valued. Where the expected term of a stock-based award does not correspond with the term for which a zero coupon interest rate is quoted, we use the nearest interest rate from the available maturities.
Expected Stock Price Volatility: We use our own historical weekly volatility because that is more reflective of market conditions.
Dividend Yield: Because we have never paid a dividend and do not expect to begin doing so in the foreseeable future, we have assumed a 0% dividend yield in valuing our stock-based awards.
Tax Effects of Stock-Based Compensation
We will only recognize a tax benefit from windfall tax deductions for stock-based awards in additional paid-in capital if an incremental tax benefit is realized after all other tax attributes currently available have been utilized.
Net Income (Loss) per Common Share
We have adopted the two class method of calculating earnings (loss) per share, due to the issuance of the Series A Preferred Stock in December 2015. Under this method, when we have a net loss we will not allocate the net loss to the holders of the Series A Preferred Stock (our participating shareholders) as they do not have a contractual obligation to share in losses. Under this method, when we have net income, we will compute net income per share using the weighted average number of common shares outstanding during the applicable period plus the weighted average number of preferred shares outstanding during the period.
Diluted net income per share is computed using the weighted average number of common shares outstanding during the applicable period, plus the dilutive effect of potential common stock. Potential common stock consists of shares issuable pursuant to stock options and warrants. Calculations of net income per share are done using the treasury stock method.
Comprehensive Income
We have presented a separate statement of other comprehensive income which includes net income, foreign currency translation adjustments and deferred gains related to derivative financial instruments. Changes in the components of AOCI are presented in the consolidated statements of redeemable convertible preferred stock and stockholders’ equity.
Recently Adopted and Issued Accounting Guidance
Adopted
In January 2017, the FASB issued ASU No. 2017-01, Business Combinations. This standard clarifies the definition of a business and provides guidance on when transactions should be accounted for as acquisitions of assets and when they should be accounted for as acquisitions of businesses. The Company early adopted this standard on July 1, 2017 and applied this guidance to the customer list that was acquired on August 1, 2017. The customer list acquired was not determined to meet the definition of a business under this standard and was therefore determined to be an asset acquisition.
In March 2016, the FASB issued Accounting Standards Update (“ASU”) No. 2016-09, Improvements to Employee Share-Based Payment Accounting. The standard update required excess tax benefits and tax deficiencies to be recorded directly through earnings as a component of income tax expense. Under previous GAAP, these differences were generally recorded in additional paid-in capital and thus had no impact on net income. The change impacted
95
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
the computation of diluted earnings per share, and the cash flows associated with those items are now classified as operating activities on the condensed statements of consolidated cash flows. Entities were permitted to make an accounting policy election for the impact of forfeitures on the recognition of expense for share-based payment awards. Forfeitures could be estimated, as required under previous GAAP, or recognized when they occur.
The Company adopted this ASU on January 1, 2017 using the transition method prescribed for each applicable provision:
|
|
• |
Based on the implementation guidance, previously unrecognized excess tax benefits should be on a modified retrospective basis beginning in the period the guidance is adopted. Accordingly, the Company recorded an increase in deferred tax assets and an offsetting cumulative-effect adjustment to retained earnings of $6.4 million as of January 1, 2017 for excess tax benefits not previously recognized. |
|
|
• |
Based on the implementation guidance, all excess tax benefits and tax deficiencies related to share based compensation will be reported in net income (loss) on a prospective basis. For the year ended December 31, 2017, $0 in income (loss) was reported. |
|
|
• |
The Company has elected to retrospectively adopt the requirement to present cash flows related to excess tax benefits as cash flows from operating activities. This adoption had no effect on cash flows for the year ended December 31, 2017, 2016 and 2015. |
|
|
• |
The Company has elected to recognize forfeitures in compensation cost as they occur. |
Issued
In August 2017 the FASB issued ASU 2017-12, Derivatives and Hedging. This standard refines hedge accounting to better align an entity’s risk management activities and financial reporting for hedging relationships through changes to both the designation and measurement guidance for qualifying hedging relationships and the presentation of hedge results. This update is effective for annual periods beginning after December 15, 2018 and interim periods within those annual periods. Early adoption is permitted. The Company does not expect the adoption of ASU 2017-12 to have a material effect on its consolidated financial statements.
In May 2017, the FASB issued ASU 2017-09, Compensation – Stock Compensation. This standard provides guidance related to the scope of stock option modification accounting, to reduce diversity in practice and reduce cost and complexity regarding existing guidance. This update is effective for annual periods beginning after December 15, 2017. Early adoption is permitted. The Company does not expect the adoption of ASU 2017-09 to have a material effect on its consolidated financial statements.
In January 2017 the FASB issued ASU No. 2017-04, Intangibles – Goodwill and Other: Simplifying the Test for Goodwill Impairment. This standard eliminates Step 2 of the goodwill impairment test. Instead, an entity should perform its annual or interim goodwill impairment test by comparing the fair value of a reporting unit with its carrying amount. An entity should recognize an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value; however, the loss recognized should not exceed the total amount of goodwill allocated to that reporting unit. This update is effective for annual and interim periods beginning after December 15, 2019. Early adoption is permitted for interim or annual goodwill impairment tests performed after January 1, 2017. The Company does not expect the adoption of ASU 2017-04 to have a material effect on its consolidated financial statements.
In August 2016, the FASB issued ASU 2016-15, Statement of Cash Flows – Classification of Certain Cash Receipts and Cash Payments. The update clarifies how specific cash receipts and cash payments are classified and presented in the statement of cash flows. This update is effective for fiscal years and interim periods within those fiscal years beginning after December 15, 2017. Early adoption is permitted. The Company does not expect the adoption of ASU 2016-15 have a material effect on its consolidated financial statements.
In February 2016, the FASB issued ASU 2016-02, Leases. The update was issued to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities, including for operating leases, on the balance sheet and disclosing key information about leasing arrangements. ASU 2016-02 is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2018. The adoption of this ASU will result in an increase on the balance sheet for lease liabilities and right to use assets. The Company is currently
96
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
evaluating the quantitative impact that adopting ASU 2016-02 will have on its consolidated financial statements and assessing any changes to its processes and controls.
In May 2014, the FASB issued ASU 2014-09, which amends FASB Accounting Standards Codification by creating Topic 606, Revenues from Contracts with Customers. This standard update calls for a number of revisions in the revenue recognition rules. In August 2015, the FASB deferred the effective date of this ASU to the first quarter of 2018, with early adoption permitted beginning in the first quarter of 2017. The ASU can be applied using a full retrospective method or a modified retrospective method of adoption. The Company has adopted this ASU on January 1, 2018 using a full retrospective method of adoption. Under this method, the Company will restate its results for each prior reporting period presented as if ASC 606 had been effective for those periods.
The adoption of this standard will require us to implement new revenue policies, procedures and internal controls related to revenue recognition. In addition, the adoption will result in enhanced financial statement disclosures surrounding the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The new standard impacts each of our two reportable segments differently due to the transactional nature of the Clinical Services Division versus the generally long-term nature of our Pharma Services Division contracts. The specific effect on our reportable segments is explained below:
Clinical Testing Revenue
Under the new standard, substantially all of our bad debt expense, which has historically been presented as part of general and administrative expense, is considered an implicit price concession and will be reported as a reduction in revenue. As a result of the new standard, there will be a material cumulative reduction in clinical revenue from previously reported periods and a similar reduction in general and administrative expenses.
Pharma Testing Revenue
The adoption of the new standard may result in changes to the timing of revenue recognition related to Pharma Services contracts as individual deliverables, for which revenue was previously recognized in the period when the deliverables were completed and invoiced, will be recognized over the remaining performance period under the new standard. Additionally, certain costs to obtain contracts, primarily for sales commissions, will be capitalized when incurred and will be amortized over the term of the contract. Under ASC 606, the Company is required to make estimates of the net sales price, including estimates of variable consideration, and recognize the estimated amount as revenue when it transfers control of the product or performance obligations to its customers. The estimation of variable consideration and the application of the related constraint, was not required under previous GAAP, variable consideration must now be determined using either an expected value or most likely amount method which requires the use of significant management judgment and estimates. The cumulative effect of this standard is not expected to result in a material change to our Pharma Services revenue.
Note C – Property and Equipment, Net
Property and equipment consisted of the following at December 31, 2017 and 2016 (in thousands):
|
|
|
2017 |
|
|
2016 |
|
|
Estimated Useful Lives in Years |
|
|||
|
Equipment |
|
$ |
33,711 |
|
|
$ |
29,220 |
|
|
3-7 |
|
|
|
Leasehold improvements |
|
|
14,517 |
|
|
|
10,550 |
|
|
2-5 |
|
|
|
Furniture and fixtures |
|
|
4,486 |
|
|
|
4,315 |
|
|
|
7 |
|
|
Computer hardware and office equipment |
|
|
10,038 |
|
|
|
8,268 |
|
|
|
3 |
|
|
Computer software |
|
|
10,331 |
|
|
|
5,346 |
|
|
3-5 |
|
|
|
Assets not yet placed in service |
|
|
3,951 |
|
|
|
3,439 |
|
|
|
— |
|
|
Subtotal |
|
|
77,034 |
|
|
|
61,138 |
|
|
|
|
|
|
Less: accumulated depreciation and amortization |
|
|
(40,530 |
) |
|
|
(27,102 |
) |
|
|
|
|
|
Property and equipment, net |
|
$ |
36,504 |
|
|
$ |
34,036 |
|
|
|
|
|
97
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
Depreciation and amortization expense on property and equipment, including leased assets in each period was as follows (in thousands):
|
|
|
For the years ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Depreciation and amortization expense |
|
$ |
15,596 |
|
|
$ |
15,937 |
|
|
$ |
6,730 |
|
In our consolidated statements of operations, we recorded depreciation and amortization expense as follows: approximately $9.3 million, $11.8 million and $4.2 million was recorded in cost of revenue for the years ended December 31, 2017, 2016 and 2015, respectively, approximately $6.2 million, $4.2 million and $2.5 million was recorded in general and administrative expenses for the years ended December 31, 2017, 2016 and 2015, respectively and approximately $96,000, $0 and $0 was recorded in research and development expense for the years ended December 31, 2017, 2016 and 2015, respectively.
Property and equipment under capital leases, included above, consists of the following at December 31, 2017 and 2016 (in thousands):
|
|
|
2017 |
|
|
2016 |
|
||
|
Equipment |
|
$ |
10,619 |
|
|
$ |
13,109 |
|
|
Furniture and fixtures |
|
|
1,012 |
|
|
|
1,081 |
|
|
Computer hardware |
|
|
4,310 |
|
|
|
5,178 |
|
|
Computer software |
|
|
607 |
|
|
|
514 |
|
|
Leasehold improvements |
|
|
1,485 |
|
|
|
69 |
|
|
Subtotal |
|
|
18,033 |
|
|
|
19,951 |
|
|
Less: accumulated depreciation and amortization |
|
|
(7,560 |
) |
|
|
(9,481 |
) |
|
Property and equipment under capital leases, net |
|
$ |
10,473 |
|
|
$ |
10,470 |
|
Note D – Acquisitions
Clarient
On December 30, 2015 (“the acquisition date”), the Company acquired from GE Medical Holding AB (“GE Medical”), a subsidiary of General Electric Company (“GE”), all of the issued and outstanding shares of common stock of Clarient, Inc., (“Clarient”) a wholly owned subsidiary of GE Medical, for a purchase price consisting of (i) cash consideration of approximately $73.8 million, which includes an approximately $6.7 million estimated working capital adjustment and adjustments for estimated cash on hand and estimated indebtedness of Clarient on the Closing Date, (ii) 15,000,000 shares of NeoGenomics’ common stock, and (iii) 14,666,667 shares of NeoGenomics’ Series A Preferred Stock pursuant to the Stock Purchase Agreement.
The cash consideration paid as part of the purchase price was funded through the following:
|
|
• |
The Company paid approximately $10.7 million using cash on hand |
|
|
• |
Approximately $9.5 million, net of transaction costs was funded using the revolving credit facility |
|
|
• |
Approximately $53.6 million, net of transaction costs was funded using the term loan |
On December 21, 2015 shareholders approved and on December 28, 2015, NeoGenomics filed with the Secretary of State of the State of Nevada amendments to its Articles of Incorporation to increase the authorized number of shares of common stock from 100.0 million shares to 250.0 million shares and to increase the authorized number of shares of preferred stock from 10.0 million shares to 50.0 million shares in order to fund the common and preferred stock portion of the purchase price.
The Company issued 15,000,000 shares of common stock as consideration for the acquisition of Clarient. The common stock includes restrictions imposed on the holder in the Investor Board Rights, Lockup and Standstill Agreement. We estimated the fair value of the common stock consideration using inputs not observable in the market and thus represents a Level 3 measurement as defined in ASC 820. The key assumption in the fair value
98
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
determination was a 15 percent discount due to lack of marketability of the common stock as a result of the restrictions imposed on the holder. The acquisition date fair value of common stock transferred is calculated below ($ in thousands, except share and per share amounts):
|
Common Stock Valuation |
|
Amount |
|
|
|
Shares of common stock issued as consideration |
|
|
15,000,000 |
|
|
Stock price per share on closing date |
|
$ |
8.04 |
|
|
Value of common stock issued as consideration |
|
$ |
120,600 |
|
|
Issue discount due to lack of marketability |
|
$ |
(18,090 |
) |
|
Fair value of common stock at December 30, 2015 |
|
$ |
102,510 |
|
The Company issued 14,666,667 shares of Series A Preferred Stock as consideration for the acquisition of Clarient. The rights of the Series A Preferred Stock are described in Note H-Class A Redeemable Convertible Preferred Stock. We estimated the fair value of the Series A Preferred Stock consideration using significant inputs not observable in the market and thus represents a Level 3 measurement as defined in ASC 820. The fair value of the Series A Preferred Stock at the acquisition date was $73.2 million or $4.99 per share. This fair value was further reduced by the intrinsic value assigned to the beneficial conversion feature to arrive at a carrying amount of $28.6 million. In December of 2016, we redeemed 8,066,667 shares of the Series A Preferred Stock, leaving 6,600,000 shares outstanding at December 31, 2016. In December of 2017, the Company issued 264,000 additional shares of Preferred Stock as a PIK dividend resulting in a balance of 6,864,000 shares outstanding as of December 31, 2017.
On a fully diluted basis, assuming full conversion of the Series A Preferred Stock, GE Medical would have owned approximately 32% of NeoGenomics at the date of issuance and approximately 27% as of December 31, 2017, after the redemption of shares. In addition, pursuant to the Investor Board Rights, Lockup and Standstill Agreement, NeoGenomics was required to appoint a director designated by GE Medical Systems to the Board.
The following table summarizes the final amounts for the fair values of the assets acquired and liabilities assumed at the acquisition date (in thousands):
|
|
|
December 30, 2015 (As Initially Reported) |
|
|
Measurement Period Adjustments |
|
|
December 30, 2015 (Final) |
|
|||
|
Current assets, including cash and cash equivalents of $890 |
|
$ |
31,978 |
|
|
$ |
672 |
|
|
$ |
32,650 |
|
|
Property and equipment |
|
|
19,241 |
|
|
|
(64 |
) |
|
|
19,177 |
|
|
Identifiable intangible assets – customer relationships |
|
|
84,000 |
|
|
|
- |
|
|
|
84,000 |
|
|
Goodwill |
|
|
143,493 |
|
|
|
598 |
|
|
|
144,091 |
|
|
Total assets acquired |
|
|
278,712 |
|
|
|
1,206 |
|
|
|
279,918 |
|
|
Current liabilities |
|
|
(12,631 |
) |
|
|
188 |
|
|
|
(12,443 |
) |
|
Deferred tax liability |
|
|
(17,904 |
) |
|
|
(964 |
) |
|
|
(18,868 |
) |
|
Long-term liabilities |
|
|
(103 |
) |
|
|
- |
|
|
|
(103 |
) |
|
Net assets acquired |
|
$ |
248,074 |
|
|
$ |
430 |
|
|
$ |
248,504 |
|
The measurement period adjustments were complete as of December 30, 2016.
Of the $84.0 million of acquired intangible assets, $81.0 million was assigned to customer relationships which are being amortized over fifteen years and $3.0 million was assigned to trade names which are being amortized over two years. We recorded approximately $7.3 million and $36 thousand of amortization expense for the years ended December 31, 2016 and 2015.
The goodwill arising from the acquisition of Clarient includes revenue synergies as a result of our existing customers and Clarient’s customers having access to each other’s testing menus and capabilities and also from the
99
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
new product lines which Clarient adds to the Company’s product portfolio. None of the goodwill is expected to be deductible for income tax purposes. The fair value of accounts receivable acquired is approximately $28.8 million.
The Company recognized acquisition related transaction costs of approximately $4.7 million during the year ended December 31, 2015. These costs include due diligence, legal, consulting and other transaction related expenses associated with the acquisition of Clarient. These expenses were included in general and administrative expenses in our consolidated statements of operations for the year ended December 31, 2015.
The amount of revenue and earnings of Clarient since the date of acquisition that are included in the consolidated statement of operations as of December 31, 2015 are as follows (in thousands):
|
|
|
For the period December 30, 2015 through December 31, 2015 |
|
|
|
Revenue |
|
$ |
665 |
|
|
Gross Margin |
|
$ |
297 |
|
|
Net Income |
|
$ |
26 |
|
The following unaudited pro forma information (in thousands) has been provided for illustrative purposes only and is not necessarily indicative of results that would have occurred had the Acquisition been in effect since January 1, 2014, nor are they necessarily indicative of future results.
|
|
|
Years ended December 31, (unaudited) |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Revenue |
|
$ |
216,029 |
|
|
$ |
214,293 |
|
|
Net (loss) attributable to common stockholders |
|
|
(71,365 |
) |
|
|
(34,084 |
) |
|
(Loss) per share |
|
$ |
(0.94 |
) |
|
$ |
(0.50 |
) |
|
Basic |
|
|
75,526 |
|
|
|
68,483 |
|
|
Diluted |
|
|
75,526 |
|
|
|
68,483 |
|
100
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The unaudited pro forma consolidated results during the years ended December 31, 2015 and 2014 have been prepared by adjusting our historical results to include the Acquisition as if it occurred on January 1, 2014. These unaudited pro forma consolidated historical results were then adjusted for the following:
|
|
• |
Remove transaction expenses from the year ended December 31, 2015 and record them in the year ended December 31, 2014 |
|
|
• |
Adjustments to reflect amortization and depreciation expense associated with the acquired assets, partially offset by the elimination of the amortization and depreciation expense associated with Clarient’s historical assets. |
|
|
• |
Removal of costs associated with MultiOmyx, assets not acquired in the transaction, and to record royalty fees due to GE Medical for continued use of the MultiOmyx product. |
|
|
• |
Remove general and administrative expenses related to a Lab Services Agreement with the Saudi Arabian National Guard Health Affairs, as GE Medical will retain this agreement. |
|
|
• |
Record interest expense under the Credit Facilities and amortization of financing costs classified as interest expense. |
|
|
• |
Remove royalty costs associated with the use of the GE brand as NeoGenomics will discontinue the use of the GE brand. |
|
|
• |
Accrue for dividends on the Series A Preferred stock and to amortize a portion of the beneficial conversion feature |
As noted above, the unaudited pro forma results of operations do not purport to be indicative of the actual results that would have been achieved by the combined company for the periods presented or that may be achieved by the combined company in the future.
Note E – Intangible Assets
In August 2017, the Company acquired a customer list from Ascend Genomics (“Ascend”) in exchange for 450,000 shares of restricted stock. See Note O to our consolidated financial statements included in this Annual Report for further disclosure. We recorded $4.1 million in intangible assets comprised of customer relationships which are being amortized over 15 years. As part of this transaction, Ascend signed a non-compete agreement which was also recorded as an intangible asset and is being amortized over 2 years.
As a result of the acquisition of Clarient in December 2015, see Note D, we recorded $84.0 million in intangible assets comprised of $81.0 million in customer relationships amortized over a fifteen year period and $3.0 million in trade name which we amortized over a two year period. Previously, we acquired Path Logic in July 2014 and recorded $1.93 million in customer relationships as an intangible asset. We were amortizing these customer relationships over a thirteen year period. In 2016, the Company determined that the Path Logic customer relationship asset was impaired and recorded an impairment loss in the amount of approximately $1.6 million. Path Logic was sold in August of 2017 and a loss of $1.1 million was recorded.
In January 2012, we entered into a Master License Agreement (the “License Agreement”) with Health Discovery Corporation, a Georgia corporation (“HDC”). We were granted an exclusive worldwide license to certain of HDC’s “Licensed Patents” and “Licensed Know-How” (as defined in the License Agreement). We have not made any milestone payments to HDC. In 2016, the Company determined that these assets were impaired and recorded an impairment loss in the amount of $1.9 million (see Note P).
101
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
Intangible assets as of December 31, 2017 and 2016 consisted of the following (in thousands):
|
|
|
|
|
December 31, 2017 |
|
|
|
|
|
|||||||||
|
|
|
Amortization Period |
|
Cost |
|
|
Accumulated Amortization |
|
|
Net |
|
|
|
|
|
|||
|
Trade Name |
|
24 months |
|
$ |
3,000 |
|
|
$ |
3,000 |
|
|
$ |
- |
|
|
|
|
|
|
Non-Compete Agreement |
|
36 months |
|
|
26 |
|
|
|
4 |
|
|
|
22 |
|
|
|
|
|
|
Customer Relationships |
|
156-180 months |
|
|
85,068 |
|
|
|
10,925 |
|
|
|
74,143 |
|
|
|
|
|
|
Total |
|
|
|
$ |
88,094 |
|
|
$ |
13,929 |
|
|
$ |
74,165 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2016 |
|
|||||||||||||
|
|
|
Amortization Period |
|
Cost |
|
|
Accumulated Amortization |
|
|
Impairment |
|
|
Net |
|
||||
|
Trade Name |
|
24 months |
|
$ |
3,000 |
|
|
$ |
1,508 |
|
|
$ |
- |
|
|
$ |
1,492 |
|
|
Customer Relationships |
|
156-180 months |
|
|
82,930 |
|
|
|
5,796 |
|
|
|
1,562 |
|
|
|
75,572 |
|
|
Support Vector Machine (SVM) technology |
|
108 months |
|
|
500 |
|
|
|
269 |
|
|
|
231 |
|
|
|
- |
|
|
Laboratory developed test (LDT) technology |
|
164 months |
|
|
1,482 |
|
|
|
524 |
|
|
|
958 |
|
|
|
- |
|
|
Flow Cytometry and Cytogenetics technology |
|
202 months |
|
|
1,000 |
|
|
|
287 |
|
|
|
713 |
|
|
|
- |
|
|
Total |
|
|
|
$ |
88,912 |
|
|
$ |
8,384 |
|
|
$ |
3,464 |
|
|
$ |
77,064 |
|
The Company recorded amortization expense of intangible assets in the consolidated statements of operations as follows (in thousands):
|
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Amortization of intangible assets |
|
$ |
6,995 |
|
|
$ |
7,272 |
|
|
$ |
412 |
|
The Company recorded amortization expense from customer relationships as a general and administrative expense. The amortization expense for the Support Vector Machine (SVM) technology, the Laboratory developed tests (LDT) technology and the Flow Cytometry and Cytogenetics technology intangibles has been recorded as research and development expense as we have not had products, services or cost savings directly attributable to these intangible assets that would require that it be recorded in cost of goods sold.
The estimated amortization expense related to amortizable intangible assets for each of the five succeeding fiscal years and thereafter as of December 31, 2017 is as follows (in thousands):
|
Years Ending December 31, |
|
As of December 31, |
|
|
|
2018 |
|
$ |
5,685 |
|
|
2019 |
|
|
5,680 |
|
|
2020 |
|
|
5,671 |
|
|
2021 |
|
|
5,671 |
|
|
2022 |
|
|
5,671 |
|
|
Thereafter |
|
|
45,787 |
|
|
Total |
|
$ |
74,165 |
|
102
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The following table summarizes the long term debt at December 31, 2017 and 2016 (in thousands):
|
|
|
2017 |
|
|
2016 |
|
||
|
Term Loan Facility |
|
$ |
71,250 |
|
|
$ |
75,000 |
|
|
Revolving Credit Facility |
|
|
25,400 |
|
|
|
22,900 |
|
|
Capital leases/loans |
|
|
10,542 |
|
|
|
10,471 |
|
|
Total Debt |
|
$ |
107,192 |
|
|
$ |
108,371 |
|
|
Less: Debt issuance costs |
|
|
(1,768 |
) |
|
|
(2,202 |
) |
|
Less: Current portion of long-term debt |
|
|
(8,989 |
) |
|
|
(8,733 |
) |
|
Total Long-Term Debt, net |
|
$ |
96,435 |
|
|
$ |
97,436 |
|
Term Loan
On December 22, 2016, the Company entered into a Credit Agreement with Regions Bank as administrative agent and collateral agent. The Credit Agreement provides for a $75.0 million term loan facility (the “Term Loan Facility”). The Credit Agreement also provides incremental facility capacity of $50 million, subject to certain conditions. On December 31, 2017, the Company had current outstanding borrowings under the Term Loan of approximately $3.7 million and long-term outstanding borrowings of approximately $66.6 million, net of unamortized debt issuance costs of $884 thousand. These costs were recorded as a reduction in the carrying amount of the related liability and are being amortized over the life of the loan.
The Term Loan Facility bears interest at a rate per annum equal to an applicable margin plus, at NeoGenomics Laboratories’ option, either (1) the Adjusted LIBOR rate for the relevant interest period, (2) an alternate base rate determined by reference to the greatest of (a) the prime lending rate of Regions, (b) the federal funds rate for the relevant interest period plus 0.5% per annum and (c) the one month LIBOR rate plus 1% per annum, or (3) a combination of (1) and (2). The applicable margin will range from 2.25% to 3.50% for LIBOR loans and 1.25% to 2.50% for base rate loans, in each case based on NeoGenomics Laboratories’ consolidated leverage ratio (as defined in the Credit Agreement). Interest on borrowings under the Revolving Credit Facility is payable on the last day of each month, in the case of each base rate loan, and on the last day of each interest period (but no less frequently than every three months), in the case of Adjusted LIBOR loans. The Company entered into an interest rate swap agreement to hedge against changes in the variable rate of a portion of this debt. See Note G-Derivative Instruments and Hedging Activities for more information on this instrument.
The Term Loan Facility and amounts borrowed under the Revolving Credit Facility are secured on a first priority basis by a security interest in substantially all of the tangible and intangible assets of NeoGenomics Laboratories and the Guarantors. The Term Loan Facility contains various affirmative and negative covenants including ability to incur liens and encumbrances; make certain restricted payments, including paying dividends on its equity securities or payments to redeem, repurchase or retire its equity securities; enter into certain restrictive agreements; make investments, loans and acquisitions; merge or consolidate with any other person; dispose of assets; enter into sale and leaseback transactions; engage in transactions with its affiliates, and materially alter the business it conducts. In addition, the Company must meet certain maximum leverage ratios and fixed charge coverage ratios as of the end of each fiscal quarter commencing with the quarter ending March 31, 2017. The Company was in compliance with all required financial covenants as of December 31, 2017.
The Term Loan Facility has a maturity date of December 21, 2021. The Credit Agreement requires NeoGenomics Laboratories to mandatorily prepay the Term Loan Facility and amounts borrowed under the Revolving Credit Facility with (i) 100% of net cash proceeds from certain sales and dispositions, subject to certain reinvestment rights, (ii) 100% of net cash proceeds from certain issuances or incurrences of additional debt, (iii) beginning with the fiscal year ending December 31, 2017, 50% of excess cash flow (as defined), subject to a step down to 0% of excess cash flow if NeoGenomics Laboratories’ consolidated leverage ratio is no greater than 2.75:1.0 and (iv) 100% of net cash proceeds from issuances of permitted equity securities by NeoGenomics Laboratories made in order to cure a failure to comply with the financial covenants. NeoGenomics Laboratories is permitted to voluntarily prepay the Term Loan Facility and amounts borrowed under the Revolving Credit Facility at any time without penalty.
103
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The Company has auto loans with various financial institutions. The auto loan terms range from 36-60 months and carry interest of 0%.
Capital Leases
The Company has entered into capital leases to purchase laboratory and office equipment. These leases expire at various dates through 2020 and the weighted average interest rate under such leases was approximately 5.20% at December 31, 2017. Most of these leases contain bargain purchase options that allow us to purchase the leased property for a minimal amount upon the expiration of the lease term. The remaining leases have purchase options at fair market value.
Property and equipment acquired under capital lease agreements (see Note C) are pledged as collateral to secure the performance of the future minimum lease payments.
Maturities of Long-Term Debt
Maturities of long-term debt at December 31, 2017 are summarized as follows (in thousands):
|
|
|
Debt |
|
|
Capital Lease Obligations & Car Loans |
|
|
Total Long Term Debt |
|
|||
|
2018 |
|
$ |
3,750 |
|
|
$ |
5,650 |
|
|
$ |
9,400 |
|
|
2019 |
|
|
5,625 |
|
|
|
4,056 |
|
|
|
9,681 |
|
|
2020 |
|
|
5,625 |
|
|
|
1,531 |
|
|
|
7,156 |
|
|
2021 |
|
|
81,650 |
|
|
|
- |
|
|
|
81,650 |
|
|
|
|
$ |
96,650 |
|
|
$ |
11,237 |
|
|
$ |
107,887 |
|
|
Less: Interest on capital leases |
|
|
- |
|
|
|
(695 |
) |
|
|
(695 |
) |
|
|
|
|
96,650 |
|
|
|
10,542 |
|
|
|
107,192 |
|
|
Less: Current portion of long-term debt |
|
|
(3,750 |
) |
|
|
(5,239 |
) |
|
|
(8,989 |
) |
|
Less: Debt issuance costs |
|
|
(1,768 |
) |
|
|
- |
|
|
|
(1,768 |
) |
|
Long-term debt, net |
|
$ |
91,132 |
|
|
$ |
5,303 |
|
|
$ |
96,435 |
|
Revolving Credit Facility
On December 22, 2016, the Company entered into a Credit Agreement with Regions Bank as administrative agent and collateral agent. The Credit Agreement provided for a $75.0 million revolving credit facility (the “Revolving Facility”). On December 31, 2017, the Company had total outstanding borrowings of approximately $24.5 million, net of unamortized debt issuance costs of $884 thousand.
The Revolving Credit Facility includes a $10 million swingline sublimit, with swingline loans bearing interest at the alternate base rate plus the applicable margin. Any principal outstanding under the Revolving Credit Facility is due and payable on December 21, 2021 or such earlier date as the obligations under the Credit Agreement become due and payable pursuant to the terms of the Credit Agreement. The Revolving Facility bears interest at a rate per annum equal to an applicable margin plus, at NeoGenomics Laboratories’ option, either (1) the Adjusted LIBOR rate for the relevant interest period, (2) an alternate base rate determined by reference to the greatest of (a) the prime lending rate of Regions, (b) the federal funds rate for the relevant interest period plus 0.5% per annum and (c) the one month LIBOR rate plus 1% per annum, or (3) a combination of (1) and (2). The applicable margin will range from 2.25% to 3.50% for Adjusted LIBOR loans and 1.25% to 2.50% for base rate loans, in each case based on NeoGenomics Laboratories’ consolidated leverage ratio. Interest on the outstanding principal of the Term Loan Facility will be payable on the last day of each month, in the case of each base rate loan, and on the last day of each interest period (but no less frequently than every three months), in the case of LIBOR loans. The Company was in compliance with all required financial covenants as of December 31, 2017.
104
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The Credit Agreement requires NeoGenomics Laboratories to mandatorily prepay the Term Loan Facility and amounts borrowed under the Revolving Credit Facility with (i) 100% of net cash proceeds from certain sales and dispositions, subject to certain reinvestment rights, (ii) 100% of net cash proceeds from certain issuances or incurrences of additional debt, (iii) beginning with the fiscal year ending December 31, 2017, 50% of excess cash flow (minus certain specified other payments), subject to a step down to 0% of excess cash flow if NeoGenomics Laboratories’ consolidated leverage ratio is no greater than 2.75:1.0 and (iv) 100% of net cash proceeds from issuances of permitted equity securities by NeoGenomics Laboratories made in order to cure a failure to comply with the financial covenants. For the year ended December 31, 2017, no excess cash flow payment was due. NeoGenomics Laboratories is permitted to voluntarily prepay the Term Loan Facility and amounts borrowed under the Revolving Credit Facility at any time without penalty, subject to customary “breakage” costs with respect to prepayments of Adjusted LIBOR rate loans made on a day other than the last day of any applicable interest period.
Note G – Derivative Instruments and Hedging Activities
Cash Flow Hedges
In December of 2016, the Company entered into an interest rate swap agreement to reduce our exposure to interest rate fluctuations on our variable rate debt obligations. This derivative financial instrument is accounted for at fair value as a cash flow hedge which effectively modifies our exposure to interest rate risk by converting a portion of our floating rate debt to a fixed rate obligation, thus reducing the impact of interest rate changes on future interest expense.
We account for derivatives in accordance with ASC Topic 815. See Note B for more information on our accounting policy related to derivative instruments and hedging activities.
Under this agreement, we receive a variable rate of interest based on LIBOR (as discussed in Note F) and we pay a fixed rate of interest at 1.59%. The interest rate swap agreement was effective as of December 30, 2016 and has a termination date of December 31, 2019. As of December 31, 2017 and 2016, the total notional amount of the Company’s interest rate swap was $50 million.
The fair value of the interest rate swap will be included in other long term assets or liabilities, when applicable. At December 31, 2017, the fair value of the derivative financial instrument was $352,000 which was included in the balance sheet as other assets and reflected in AOCI. At December 31, 2016, it was determined that the fair value of this instrument was not significant and therefore is not recorded on the balance sheet as an asset or liability, nor is the change in value reflected through AOCI. The instrument will be evaluated on a monthly basis and resulting increases or decreases will be recorded as a component of AOCI and will be reclassified to interest expense in the period during which the hedged transaction affects earnings. Cash flows from the interest rate swap are to be included in operating activities on the consolidated statement of cash flows. As the specific terms and notional amounts of the derivative financial instrument match those of the fixed-rate debt being hedged, the derivative instrument is assumed to be a perfectly effective hedge and accordingly, there is no impact to the Company's consolidated statements of operations. As of December 31, 2017, the Company estimates that it will reclassify gains or losses on derivative instruments of $115,000 from AOCI to earnings during the next twelve months as the anticipated cash flows occur.
Note H – Class A Redeemable Convertible Preferred Stock
On December 30, 2015, (“Original Issue Date”), the Company issued 14,666,667 shares of its Series A Redeemable Convertible Preferred stock (“Series A Preferred Stock”) as part of the consideration given to acquire all of the outstanding stock of Clarient Inc. (see Note D). The Series A Preferred Stock has a face value of $7.50 per share for a total liquidation value of $110 million. The Company recorded the Series A Preferred Stock on the Original Issue Date at fair value of approximately $73.2 million or $4.99 per share, net of the $36.8 million discount to the liquidation value. The $36.8 million discount relates to the rights and features (listed below) of the Series A Preferred Stock. Additionally, the fair value of the common stock into which the Series A Preferred Stock was convertible at the Original Issue Date exceeded the allocated purchase price fair value of the Series A Preferred Stock by approximately $44.7 million on the date of issuance, resulting in a beneficial conversion feature (“BCF”).
105
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The Original Issue Date fair value of $73.2 million was further reduced by $44.7 million allocated to the value of the BCF, resulting in a carrying value on Original Issue Date of $28.5 million. The Series A Preferred Stock will accrue dividends at an increasing rate as described below. Since the dividends accrue at an escalating rate the Company records deemed dividends using the effective interest method starting from the Original Issue Date.
The Company classified the Series A Preferred Stock as temporary equity on the consolidated balance sheets due to certain change in control events that are outside the Company’s control, including deemed liquidation events described below.
On December 22, 2016, the Company redeemed 8,066,667 shares of the Series A Preferred Stock for $55.0 million in cash. The redemption amount per share equaled $6.82 ($7.50 minus the liquidation discount of 9.09%). At December 31, 2016, following the redemption, 6,600,000 shares of Series A Preferred Stock were outstanding. In December 2017, the Company issued 264,000 additional shares of Preferred Stock as a Paid-in-Kind (“PIK”) dividend, resulting in a balance of 6,864,000 shares outstanding as of December 31, 2017.
The shares of Series A Preferred Stock have the following rights and features:
Rank
The Series A Preferred Stock ranks senior to all other classes and series of our capital stock, including our common stock and other series of preferred stock (collectively, “Junior Stock”) that we may issue in the future, including with respect to dividend and other distribution rights or rights upon a liquidation event as defined.
Voting Rights
Each holder of Series A Preferred Stock has such number of votes for each share of Series A Preferred Stock held of record by such holder on an as-converted (into common stock) basis, on each matter upon which holders of common stock have the right to vote and will vote together with the holders of common stock (and any other class or series which may be similarly entitled to vote) as one class on all matters upon which holders of common stock have the right to vote, and not as a separate class or series other than as set forth below.
In addition to any other vote of our stockholders required under applicable law, if any shares of Series A Preferred Stock remain outstanding at any point in time, the affirmative vote or written consent of the holders of at least a majority of the then issued and outstanding shares of Series A Preferred Stock, voting together as a single class, will be required for us to effect any corporate action (whether taken by amendment, merger, consolidation or otherwise) to:
|
|
• |
increase or decrease the authorized number of shares of Series A Preferred Stock; |
|
|
• |
create or authorize the creation of or issue any equity security, including any security convertible into or exchangeable for any equity security, of any other class or series having rights, preferences or privileges ranking on parity with or senior to or prior to the Series A Preferred Stock; |
|
|
• |
change the powers, designations, preferences, limitations, restrictions, voting or other rights of the Series A Preferred Stock set forth in the Certificate of Designations; |
|
|
• |
alter or amend any provision of our Articles of Incorporation or Bylaws in a manner adverse to the rights of the Series A Preferred Stock set forth in the Certificate of Designations; |
|
|
• |
redeem, repurchase or otherwise acquire any Junior Stock, except for repurchases of Junior Stock held by our employees, independent contractors, consultants or medical doctors upon termination of their employment or services pursuant to employment agreements, consulting agreements or settlement agreements providing for such repurchase; |
|
|
• |
issue any additional shares of Series A Preferred Stock, except as required pursuant to the terms of the Certificate of Designations; |
|
|
• |
effect an exchange, reclassification or cancellation of all or part of the Series A Preferred Stock; or |
106
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
|
|
• |
change the Series A Preferred Stock into the same or a different number of shares, with or without par value, of the same or another class. |
Dividends
Commencing on the one year anniversary of the Original Issue Date and ending on the date on which the Series A Preferred Stock automatically converts as described below, in the event that any shares of Series A Preferred Stock remain issued and outstanding, dividends (the “PIK Dividends”) on each share of Series A Preferred Stock will accrue quarterly in arrears on the last day of each March, June, September and December, and in kind in an amount of shares of Series A Preferred Stock equal to (a) the product of the PIK Dividend rate described in the table below for the period indicated, multiplied by the then effective Liquidation Preference, as defined, per share of Series A Preferred Stock, divided by (b) four.
|
|
|
|
|
|
|
For the Period: |
|
PIK Dividend Rate |
|
|
|
Commencing on the Original Issue Date and ending on the 1st anniversary of the Original Issue Date |
|
|
0.0 |
% |
|
Commencing on the day after the 1st anniversary of the Original Issue Date and ending on the 4th anniversary of the Original Issue Date |
|
|
4.0 |
% |
|
Commencing on the day after the 4th anniversary of the Original Issue Date and ending on the 5th anniversary of the Original Issue Date |
|
|
5.0 |
% |
|
Commencing on the day after the 5th anniversary of the Original Issue Date and ending on the 6th anniversary of the Original Issue Date |
|
|
6.0 |
% |
|
Commencing on the day after the 6th anniversary of the Original Issue Date and ending on the 7th anniversary of the Original Issue Date |
|
|
7.0 |
% |
|
Commencing on the day after the 7th anniversary of the Original Issue Date and ending on the 8th anniversary of the Original Issue Date |
|
|
8.0 |
% |
|
Commencing on the day after the 8th anniversary of the Original Issue Date and ending on the 9th anniversary of the Original Issue Date |
|
|
9.0 |
% |
|
Commencing on the day after the 9th anniversary of the Original Issue Date and ending on the date of automatic conversion |
|
|
10.0 |
% |
The PIK Dividends are cumulative and accrue whether or not they have been earned or declared and whether or not there are profits, surplus or other funds of NeoGenomics Laboratories legally available for the payment of PIK Dividends. On December 31 of each year, beginning on the first anniversary of the Original Issue Date and ending on the date on which the Series A Preferred Stock automatically converts as described below, all PIK Dividends which have accrued on a share of Series A Preferred Stock outstanding during such calendar year (or such shorter period in the case of the initial period) will be added to the then effective Liquidation Preference of such share of Series A Preferred Stock. In the event of a redemption or conversion of the Series A Preferred Stock or a Liquidation Event on any date other than December 31 of any calendar year, the redemption amount payable upon a redemption, the Liquidation Preference and the shares of Series A Preferred Stock so convertible in connection therewith, as applicable, will be increased by PIK Dividends in an amount equal to the Liquidation Preference multiplied by the product of (a) the PIK Dividend rate in effect for such year reflected in the table above, and (b) the quotient of (x) the number of calendar days elapsed from January 1 of such year to the date of consummation of such redemption, conversion or Liquidation Event, as applicable, divided by (y) 360.
If, on account of an increase in the Liquidation Preference of a share of Series A Preferred Stock pursuant to the preceding paragraph, any holder of Series A Preferred Stock would be prohibited by any applicable law, rule or regulation from holding its Series A Preferred Stock or converting all of its Series A Preferred Stock at the then effective conversion price, without receiving the consent of any governmental authority that has not been obtained at such time, then the Liquidation Preference will not be increased, and such PIK Dividend will be paid in cash in lieu of such increase in the Liquidation Preference. If the condition set forth above ceases to exist prior to the date of an optional conversion or the date of the automatic conversion of the Series A Preferred Stock, the Liquidation Preference will be increased to such Liquidation Preference that would then be in effect as if such condition had not
107
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
existed. Had none of the 14,666,667 shares of Series A Preferred Stock been redeemed prior to automatic conversion into our common stock on the tenth anniversary of closing, we would have been required to issue an additional 10,775,454 shares of Series A Preferred Stock as PIK Dividends. If the remaining 6,864,000 shares of Series A Preferred Stock remains outstanding until the automatic conversion date we will be required to issue an additional 4,848,955 shares of Series A Preferred Stock as PIK Dividends prior to the automatic conversion.
Liquidation, Dissolution or Winding-up; Liquidation Preference
To the extent not prohibited by applicable law, upon the occurrence of any Liquidation Event, each holder of Series A Preferred Stock will be entitled to receive, prior and in preference to any distribution of any of the assets or funds of NeoGenomics to the holders of shares of Junior Stock out of the assets of NeoGenomics legally available therefor, whether such assets are capital, surplus or earnings, an amount, payable in cash, equal to $7.50 plus all declared and unpaid dividends thereon, including all accrued and unpaid PIK Dividends regardless of whether there has been any payment-in-kind with respect thereto and after giving effect to the second paragraph under “—Dividends”, in each case, as adjusted for any stock dividends, combinations, splits, recapitalizations and similar events with respect to such shares (the “Liquidation Preference”), for each share of Series A Preferred Stock held by such holder. “Liquidation Event” means any liquidation, dissolution or winding up of the Company, either voluntary or involuntary, and any Deemed Liquidation Event.
A Deemed Liquidation Event includes any of the following: (a) the acquisition by any person other than a holder of Series A Preferred Stock or an affiliate thereof of 50% or more of our voting securities; (b) any consolidation or merger of NeoGenomics with or into any other corporation or other entity or person, or any other corporate reorganization, in which our stockholders immediately prior to such consolidation, merger or reorganization, own less than 50% of our voting power immediately after such consolidation, merger or reorganization; and (c) any sale, lease, license, transfer or other disposition of all or substantially all of the assets, technology or intellectual property of NeoGenomics, other than non-exclusive licenses granted in the ordinary course of our business.
Automatic Conversion
Each share of Series A Preferred Stock issued and outstanding as of the tenth anniversary of the Original Issue Date will automatically convert into fully paid and non-assessable shares of common stock. The number of shares of common stock to which a holder of Series A Preferred Stock will be entitled upon conversion will be equal to the quotient of the then effective Liquidation Preference, divided by the then effective conversion price. The conversion price will be equal to $7.50, multiplied by the conversion rate, which will initially be equal to 1.0, but is subject to anti-dilution adjustments that may occur prior to the date of the automatic conversion.
Optional Conversion by Holder
At any time, from and after the third anniversary of the Original Issue Date, to the extent the VWAP of our common stock equals or exceeds $8.00 per share, as adjusted for any stock dividends, combinations, splits, recapitalizations and similar events with respect to shares of our common stock, for 30 consecutive trading days, any holder, upon written notice, will have the right to convert any or all shares of Series A Preferred Stock it owns into fully paid and non-assessable shares of common stock. The number of shares of common stock to which a holder of Series A Preferred Stock will be entitled upon conversion will be equal to the quotient of the then effective Liquidation Preference, divided by the then effective conversion price, and the date upon which we receive the holder’s notice of conversion will be the effective date of any optional conversion. For purposes of the foregoing, “VWAP” means, as of any applicable date of determination, the volume weighted average per share price of shares of our common stock on the applicable trading day on the principal national securities exchange on which our common stock is listed or admitted to trading.
Conversion Rate and Conversion Price
The conversion price for the Series A Preferred Stock is $7.50 per share, multiplied by the then effective conversion rate. The conversion rate in effect for conversion of each share of Series A Preferred Stock into common stock is
108
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
initially be 1.0, subject to adjustments for stock splits, reclassifications and certain distributions and as described under “—Reorganizations, Mergers and Consolidations”.
No Fractional Shares
We are not required to issue or cause to be issued fractional shares of common stock pursuant to any provision of the Certificate of Designations. If any fraction of a share of common stock would be issuable pursuant to the Certificate of Designations, the number of shares of common stock to be issued will be rounded up to the nearest whole share.
Reorganizations, Mergers and Consolidations
In case of any consolidation or merger of NeoGenomics with any other entity (other than a wholly owned subsidiary of NeoGenomics), or in case of any sale or transfer of all or substantially all of our assets, or in case of any share exchange pursuant to which all of the outstanding shares of common stock are converted into other securities or property of NeoGenomics, we will, prior to or at the time of such transaction, make appropriate provision or cause appropriate provision to be made so that holders of each share of Series A Preferred Stock then outstanding will have the right thereafter to convert such shares of Series A Preferred Stock into the kind and amount of shares of stock and other securities and property receivable upon such consolidation, merger, sale, transfer or share exchange by a holder of the number of shares of common stock into which such share of Series A Preferred Stock could have been converted immediately prior to the effective date of such consolidation, merger, sale, transfer or share exchange. If in connection with any such consolidation, merger, sale, transfer or share exchange, each holder of shares of common stock is entitled to elect to receive either securities, cash or other assets upon completion of such transaction, we will provide or cause to be provided to each holder of Series A Preferred Stock the right to elect the securities, cash or other assets into which the Series A Preferred Stock held by such holder will be convertible after consummation of any such transaction on the same terms and subject to the same conditions applicable to holders of the common stock.
Prohibitions on Transfers
No sale, exchange, delivery, assignment, transfer, disposal, encumbrance, pledge or hypothecation, whether voluntary, involuntary, by operation of law, or resulting from death, disability or otherwise may be made by a holder of any shares of Series A Preferred Stock without our express written consent, except that a holder may transfer shares of Series A Preferred Stock to an affiliate of such holder upon written notice to us.
Amendments; Modifications
No provision of the Certificate of Designations may be amended, except in a written instrument signed by NeoGenomics and holders of at least a majority of the shares of Series A Preferred Stock then outstanding.
Redemption at the Option of the Company
At any time, and from time to time, we may redeem for cash all, or any portion of, the outstanding Series A Preferred Stock at a price per share equal to the then effective Liquidation Preference, provided the aggregate amount redeemed at such time is not less than (a) from the Original Issue Date until the fourth anniversary thereof, $10.0 million and (b) thereafter, $5.0 million, and in each case only in $1.0 million increments above such amounts. The amount payable by us in the event of a redemption during the period from the Original Issue Date until the fourth anniversary thereof will be discounted as set forth below under “—Redemption Discounts”.
Redemption at the Option of the Holder upon Future Capital Raise
For so long as any shares of Series A Preferred Stock remain outstanding, in the event that we issue any other class or series of equity or common stock equivalents or any unsecured debt securities for cash consideration, we are required to apply at least 50% of the net cash proceeds from any such issuance to redeem shares of Series A Preferred Stock for cash at a redemption price per share equal to the then effective Liquidation Preference. Cash proceeds received by us in connection with the exercise of options, warrants or similar securities that we issued to
109
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
our employees, directors independent contractors, consultants or medical doctors as compensation will not be applied to the redemption of shares of Series A Preferred Stock. The amount payable by us in the event of a redemption during the period from the Original Issue Date until the fourth anniversary thereof will be discounted as set forth below under “—Redemption Discounts”.
Redemption Discounts
Commencing on the Original Issue Date and ending on the fourth anniversary thereof, in the event that any shares of Series A Preferred Stock are redeemed, the amount payable by us for each share being redeemed will be reduced by an amount determined by multiplying the discount rate listed below for the period in which the redemption is consummated by the then effective Liquidation Preference before such discount is applied.
|
For the Period: |
|
Discount |
|
|
|
Commencing on the Original Issue Date and ending on the 1st anniversary of the Original Issue Date |
|
|
9.0909 |
% |
|
Commencing on the day after the 1st anniversary of the Original Issue Date and ending on the 2nd anniversary of the Original Issue Date |
|
|
6.8182 |
% |
|
Commencing on the day after the 2nd anniversary of the Original Issue Date and ending on the 3rd anniversary of the Original Issue Date |
|
|
4.5455 |
% |
|
Commencing on the day after the 3rd anniversary of the Original Issue Date and ending on the 4th anniversary of the Original Issue Date |
|
|
2.2727 |
% |
From and after the fourth anniversary of the Original Issue Date, no reduction will be made for any amount payable in connection with a redemption.
The 10 year liquidation value of the Series A Preferred Stock is as follows (in thousands except share amounts):
The fair value of the Series A Preferred Stock originally issued was being accreted to the ten year liquidation value of $190.8 million using an effective interest rate of approximately 10.06% as follows (in thousands):
|
|
|
Anniversary of Closing Date - Original Issuance on 14,666,667 shares |
|
|||||||||||||||||||||||||||||||||||||
|
|
|
Year 1 |
|
|
Year 2 |
|
|
Year 3 |
|
|
Year 4 |
|
|
Year 5 |
|
|
Year 6 |
|
|
Year 7 |
|
|
Year 8 |
|
|
Year 9 |
|
|
Year 10 |
|
||||||||||
|
Carrying Value |
|
$ |
73,200 |
|
|
$ |
80,560 |
|
|
$ |
88,661 |
|
|
$ |
97,576 |
|
|
$ |
107,387 |
|
|
$ |
118,185 |
|
|
$ |
130,069 |
|
|
$ |
143,147 |
|
|
$ |
157,541 |
|
|
$ |
173,382 |
|
|
Deemed Dividends |
|
|
7,360 |
|
|
|
8,101 |
|
|
|
8,915 |
|
|
|
9,811 |
|
|
|
10,798 |
|
|
|
11,884 |
|
|
|
13,078 |
|
|
|
14,394 |
|
|
|
15,841 |
|
|
|
17,434 |
|
|
|
|
$ |
80,560 |
|
|
$ |
88,661 |
|
|
$ |
97,576 |
|
|
$ |
107,387 |
|
|
$ |
118,185 |
|
|
$ |
130,069 |
|
|
$ |
143,147 |
|
|
$ |
157,541 |
|
|
$ |
173,382 |
|
|
$ |
190,816 |
|
After redemption, the fair value of the remaining Series A Preferred Stock will be accreted to the ten year liquidation value of $85.9 million using an effective interest rate of approximately 10.06% as follows (in thousands):
|
|
|
Anniversary of Closing Date - Post Redemption on 6,600,000 shares remaining |
|
|||||||||||||||||||||||||||||||||||||
|
|
|
Year 1 |
|
|
Year 2 |
|
|
Year 3 |
|
|
Year 4 |
|
|
Year 5 |
|
|
Year 6 |
|
|
Year 7 |
|
|
Year 8 |
|
|
Year 9 |
|
|
Year 10 |
|
||||||||||
|
Fair Value |
|
$ |
32,940 |
|
|
$ |
36,252 |
|
|
$ |
39,897 |
|
|
$ |
43,909 |
|
|
$ |
48,324 |
|
|
$ |
53,183 |
|
|
$ |
58,531 |
|
|
$ |
64,416 |
|
|
$ |
70,893 |
|
|
$ |
78,021 |
|
|
Deemed Dividends |
|
|
3,312 |
|
|
|
3,645 |
|
|
|
4,012 |
|
|
|
4,415 |
|
|
|
4,859 |
|
|
|
5,348 |
|
|
|
5,885 |
|
|
|
6,477 |
|
|
|
7,128 |
|
|
|
7,846 |
|
|
|
|
$ |
36,252 |
|
|
$ |
39,897 |
|
|
$ |
43,909 |
|
|
$ |
48,324 |
|
|
$ |
53,183 |
|
|
$ |
58,531 |
|
|
$ |
64,416 |
|
|
$ |
70,893 |
|
|
$ |
78,021 |
|
|
$ |
85,867 |
|
110
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The fair value of the common stock into which the Series A Preferred Stock was convertible at the date of issuance exceeded the allocated purchase price fair value of the Series A Preferred Stock by approximately $44.7 million on the date of issuance, resulting in a beneficial conversion feature. The calculation of the Beneficial Conversion Feature at the Original Issue Date is as follows (in thousands except share and per share amounts):
|
Original Issue Date fair value of Series A Preferred Stock |
|
$ |
73,200 |
|
|
Common shares the stock converts into |
|
|
14,666,667 |
|
|
Common shares the stock coverts into |
|
|
14,666,667 |
|
|
Excess fair value of stock over conversion price |
|
$ |
3.05 |
|
|
Effective conversion price |
|
$ |
4.99 |
|
|
Value of beneficial conversion feature |
|
$ |
44,720 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stock price on issue date |
|
$ |
8.04 |
|
|
Fair Value of Series A Preferred Stock |
|
$ |
73,200 |
|
|
Effective conversion price |
|
$ |
4.99 |
|
|
Value of Beneficial Conversion Feature |
|
$ |
(44,720 |
) |
|
Excess fair value over conversion price |
|
$ |
3.05 |
|
|
Carrying Value of Series A Preferred stock at issuance |
|
$ |
28,480 |
|
After the redemption of 8,066,667 shares of the Series A Preferred Stock, the remaining BCF allocated to the 6,600,000 shares outstanding is approximately $20.1 million (6,660,000 * $3.05). Since the Series A Preferred Stock first becomes convertible three years from the Original Issuance Date the Company is recognizing the BCF as non-cash deemed dividends of approximately $6.7 million per year in each of the first three years the Series A Preferred Stock is outstanding.
In addition to the BCF recorded at the Original Issue Date, we are required to record additional BCF discounts upon the issuance of PIK shares issued quarterly, as dividends, starting after the first year from the Original Issue Date. After the early redemption, the face value of the remaining Series A Preferred Stock is $49.5 million and 264,000 ($49.5 million * 4.0%) / $7.50) additional shares of Series A Preferred Stock were issued for the first year dividends payable. Using the same calculations as the table above, the additional 264,000 shares are discounted by a BCF of approximately $0.8 million, which is being amortized over the remaining period up to the earliest conversion date, which is three years from the Original Issue Date.
111
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The following table details the amounts recorded for the components of the Series A Preferred stock separately for the shares redeemed and for the shares remaining after redemption in 2016:
|
Reconciliation of amounts recorded |
|
Original Issue Date |
|
|
Redeemed Shares |
|
|
Shares Remaining |
|
|
Income Statement Impact |
|
||||
|
Shares of Series A Preferred Stock |
|
|
14,666,667 |
|
|
|
8,066,667 |
|
|
|
6,600,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Face (conversion) value |
|
$ |
110,000 |
|
|
$ |
60,500 |
|
|
$ |
49,500 |
|
|
$ |
- |
|
|
Issue discount |
|
|
(36,800 |
) |
|
|
(20,240 |
) |
|
|
(16,560 |
) |
|
|
- |
|
|
Beneficial conversion feature |
|
|
(44,720 |
) |
|
|
(24,596 |
) |
|
|
(20,124 |
) |
|
|
- |
|
|
Carrying value at Original Issue Date |
|
|
28,480 |
|
|
|
15,664 |
|
|
|
12,816 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deemed dividends expense recorded |
|
|
7,360 |
|
|
|
4,048 |
|
|
|
3,312 |
|
|
|
7,360 |
|
|
Amortization expense of BCF |
|
|
14,988 |
|
|
|
8,244 |
|
|
|
6,745 |
|
|
|
14,988 |
|
|
Carrying value pre-redemption |
|
$ |
50,828 |
|
|
$ |
27,956 |
|
|
$ |
22,873 |
|
|
$ |
22,348 |
|
|
Accelerate discount expense on redeemed shares |
|
|
|
|
|
|
18,973 |
|
|
|
- |
|
|
|
18,973 |
|
|
Remove deemed dividends not payable on redeemed shares |
|
|
|
|
|
|
(2,781 |
) |
|
|
- |
|
|
|
(2,781 |
) |
|
Remove BCF not realized by holder |
|
|
|
|
|
|
(8,244 |
) |
|
|
- |
|
|
|
(8,244 |
) |
|
Add original amount of BCF back to Series A Preferred Stock from APIC |
|
|
|
|
|
|
24,596 |
|
|
|
- |
|
|
|
- |
|
|
Record cash paid at redemption of $55.0 million |
|
|
|
|
|
|
(60,500 |
) |
|
|
- |
|
|
|
(5,500 |
) |
|
Carrying value after redemption at December 31, 2016 |
|
|
|
|
|
|
- |
|
|
|
22,873 |
|
|
|
- |
|
|
Cumulative impact on income statement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24,796 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amounts from deemed dividends and discounts |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
18,051 |
|
|
Amounts from BCF |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6,745 |
|
To calculate any gain or loss realized on the redemption of the Series A Preferred Stock, the Company took the carrying value of the shares of Series A Preferred Stock before the redemption and added the amount of the beneficial conversion feature originally recorded with the redeemed shares and compared that total to the consideration being paid, in this case the $55 million. The following table summarizes the calculation of the net loss realized upon the redemption of the 8,066,667 shares of Series A Preferred Stock in 2016 which agrees with the cumulative impact on the income statement in the table above.
|
Calculation of loss on redemption |
|
|
|
|
|
Carrying value pre-redemption on shares redeemed |
|
$ |
27,956 |
|
|
Value of original BCF on redeemed shares |
|
|
24,596 |
|
|
|
|
|
52,552 |
|
|
Cash paid to redeem shares |
|
|
55,000 |
|
|
Loss on redemption of Series A Preferred Stock |
|
|
(2,448 |
) |
|
|
|
|
|
|
|
Income statement expense pre-redemption |
|
|
22,348 |
|
|
Plus: Loss on redemption of Series A Preferred Stock |
|
|
2,448 |
|
|
Cumulative income statement impact as of December 31, 2017 |
|
$ |
24,796 |
|
112
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
On December 22, 2017, the United States enacted the Tax Cuts and Jobs Act. The Act makes significant modifications to the provisions of the Internal Revenue Code, including but not limited to, a corporate tax rate decrease from 35% to 21% effective as of January 1, 2018. The Company’s net deferred tax assets and liabilities have been revalued at the newly enacted U.S. corporate rate in the year of enactment. The adjustment related to the remeasurement of the deferred tax asset and liability balances, including the revaluation of amounts originally reported in other comprehensive income, is a net benefit of $3.0 million and is included in income as of December 31, 2017.
Significant components of the provision for income taxes for the years ended December 31, 2017, 2016 and 2015 are as follows (in thousands):
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Current: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
(91 |
) |
|
$ |
(8 |
) |
|
$ |
56 |
|
|
State |
|
|
14 |
|
|
|
39 |
|
|
|
101 |
|
|
Total Current Provision (Benefit) |
|
$ |
(77 |
) |
|
$ |
31 |
|
|
$ |
157 |
|
|
Deferred: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal |
|
$ |
(2,739 |
) |
|
$ |
(1,451 |
) |
|
$ |
(1,866 |
) |
|
State |
|
|
297 |
|
|
|
(281 |
) |
|
|
(245 |
) |
|
Foreign |
|
|
(115 |
) |
|
|
- |
|
|
|
- |
|
|
Total Deferred Provision (Benefit) |
|
$ |
(2,557 |
) |
|
$ |
(1,732 |
) |
|
$ |
(2,111 |
) |
A reconciliation of the differences between the effective tax rate and the federal statutory tax rate for the years ended December 31, 2017, 2016 and 2015 is as follows:
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Federal statutory tax rate |
|
|
34.00 |
% |
|
|
34.00 |
% |
|
|
34.00 |
% |
|
State income taxes, net of federal income tax benefit |
|
|
(3.78 |
)% |
|
|
3.43 |
% |
|
|
4.41 |
% |
|
Non-deductible expenses |
|
|
(13.38 |
)% |
|
|
(1.88 |
)% |
|
|
(29.17 |
)% |
|
Non-deductible stock options and warrants |
|
|
(19.76 |
)% |
|
|
(13.37 |
)% |
|
|
(10.24 |
)% |
|
Prior year adjustments for stock compensation |
|
|
— |
|
|
|
— |
|
|
|
(0.26 |
)% |
|
Deferred revaluation for Tax Cuts and Jobs Act |
|
|
88.53 |
% |
|
|
|
|
|
|
|
|
|
Foreign Tax Rate Differential |
|
|
(9.89 |
)% |
|
|
|
|
|
|
|
|
|
Other, net |
|
|
(0.02 |
%) |
|
|
0.73 |
% |
|
|
— |
% |
|
Valuation allowance |
|
|
— |
|
|
|
— |
|
|
|
49.49 |
% |
|
Effective tax rate |
|
|
75.70 |
% |
|
|
22.91 |
% |
|
|
48.23 |
% |
At December 31, 2017 and 2016, our current and non-current deferred income tax assets and liabilities consisted of the following (in thousands):
|
|
|
2017 |
|
|
2016 |
|
||
|
Deferred income tax assets (liabilities): |
|
|
|
|
|
|
|
|
|
Allowance for doubtful accounts |
|
$ |
170 |
|
|
$ |
340 |
|
|
Accrued vacation |
|
|
943 |
|
|
|
759 |
|
|
Other accruals |
|
|
84 |
|
|
|
84 |
|
|
Other |
|
|
107 |
|
|
|
66 |
|
|
Net operating loss carry-forwards |
|
|
12,282 |
|
|
|
12,222 |
|
|
AMT credit carry-forward |
|
|
- |
|
|
|
144 |
|
|
Nonqualified stock options and warrants |
|
|
1,342 |
|
|
|
1,264 |
|
|
Accumulated depreciation and amortization |
|
|
(21,235 |
) |
|
|
(29,852 |
) |
|
Net deferred income tax liabilities |
|
$ |
(6,307 |
) |
|
$ |
(14,973 |
) |
113
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
At December 31, 2017, the Company had federal net operating loss carry forwards of approximately $50.2 million and state net operating loss carry forwards of approximately $22.6 million. The Company adopted ASU 2016-09 as of January 1, 2017. Adoption requires a modified retrospective transition whereby the cumulative-effect is an adjustment to equity as of the beginning of the period. This resulted in an adjustment to the deferred tax assets related to net operating loss carryforwards and equity of $6.4 million. Assuming our net operating loss carry forwards are not disallowed because of certain “change in control” provisions of the Internal Revenue Code, these net operating loss carry forwards expire in various years beginning in the year ending December 31, 2029.
Management assesses the available positive and negative evidence to estimate if sufficient future taxable income will be generated to use the existing deferred tax assets. We previously established a valuation allowance to fully reserve our net deferred income tax assets as such assets did not meet the more likely than not recognition standard established by ASC Topic 740. As of December 31, 2015, due to an increase of deferred tax liabilities resulting from the acquisition of Clarient, management has determined that sufficient positive evidence exists to conclude that it is more likely than not that additional deferred taxes are realizable and therefore reduced the valuation allowance to zero. Our valuation allowance decreased by approximately $0, $0 and $2,240,800 during the years ended December 31, 2017, 2016 and 2015, respectively.
We file income tax returns in the U.S. federal jurisdiction and in various state jurisdictions. Tax regulations within each jurisdiction are subject to the interpretation of the related tax laws and regulations and require significant judgment. For federal and state purposes, we have open tax years ending December 31, 2009 to December 31, 2017. We are not currently subject to any ongoing income tax examinations.
We have examined our current and past tax positions taken, and have concluded that it is more likely than not these tax positions will be sustained in the event of an examination and that there would be no material impact to our effective tax rate. As of December 31, 2017 we had no unrecognized tax benefits. In the event interest or penalties will be accrued, our policy is to include these amounts related to unrecognized tax benefits in income tax expense. As of December 31, 2017, we had no accrued interest or penalties related to uncertain tax positions.
Note J – Net (Loss) per Share
The following table provides the computation of basic and diluted net (loss) per share for the years ended December 31, 2017, 2016 and 2015 (in thousands, except share and per share amounts):
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Net (loss) |
|
$ |
(846 |
) |
|
$ |
(5,723 |
) |
|
$ |
(2,535 |
) |
|
Deemed dividends on preferred stock |
|
|
3,645 |
|
|
|
18,011 |
|
|
|
40 |
|
|
Amortization of preferred stock beneficial conversion feature |
|
|
6,902 |
|
|
|
6,663 |
|
|
|
82 |
|
|
Net (loss) available to common stockholders |
|
$ |
(11,393 |
) |
|
$ |
(30,397 |
) |
|
$ |
(2,657 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic weighted average common shares outstanding |
|
|
79,426 |
|
|
|
77,542 |
|
|
|
60,526 |
|
|
Effect of potentially dilutive securities |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
Diluted weighted average shares outstanding |
|
|
79,426 |
|
|
|
77,542 |
|
|
|
60,526 |
|
|
Basic net (loss) per share attributable to common stockholders |
|
$ |
(0.14 |
) |
|
$ |
(0.39 |
) |
|
$ |
(0.04 |
) |
|
Diluted net (loss) per share attributable to common stockholders |
|
$ |
(0.14 |
) |
|
$ |
(0.39 |
) |
|
$ |
(0.04 |
) |
We have adopted the two class method in calculating earnings per share as we have determined our preferred shares to be participating securities. Under this method, we have included in weighted average shares outstanding all of our preferred shares as we have assumed conversion to common shares. We have not allocated the net loss to our participating shareholders as they do not have a contractual obligation to share in losses.
114
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
For the years ended December 31, 2017, 2016 and 2015, 1.6 million, 1.7 million and 103,000 options were excluded from the calculation of diluted earnings per share because the effect of including these potential shares was anti-dilutive. The impact of contingently convertible Series A Preferred Stock was excluded from the calculation of diluted earnings per share because the effect of including these potential shares was anti-dilutive.
Note K – Stock Options, Stock Purchase Plan and Warrants
Stock Option Plan
On May 25, 2017, the board of directors of Parent (the “Board of Directors”) further amended the Equity Incentive Plan, originally effective as of October 14, 2003, and previously amended and restated effective as of October 31, 2006, April 16, 2013, May 4, 2015 and December 21, 2015. The Amended Plan allows for the award of equity incentives, including stock options, stock appreciation rights, restricted stock awards, stock bonus awards, deferred stock awards, and other stock-based awards to certain employees, directors, or officers of, or key non-employee advisers or consultants, including contracted physicians to the Company or its subsidiaries. The Amended Plan, provides that the maximum aggregate number of shares of the Company’s common stock reserved and available for issuance under the Amended Plan is 18,650,000.
As of December 31, 2017 and 2016, option and stock awards outstanding totaled 6,342,526 and 5,136,110 shares, respectively. The outstanding options in 2016 include 200,000 options issued outside of the Amended Plan to Douglas VanOort, the Company’s Chairman and Chief Executive Officer. As of December 31, 2017 and 2016, a total of approximately 5,440,222 and 1,670,205 shares, respectively, were available for future option and stock awards under the Amended Plan. Options typically expire after 5 years and generally vest over 3 or 4 years, but each grant’s expiration, vesting and exercise price provisions are determined at the time the awards are granted by the Compensation Committee of the Board of Directors.
The fair value of each stock option award granted during the years ended December 31, 2017, 2016 and 2015 was estimated as of the grant date using a trinomial lattice model with the following weighted average assumptions:
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Expected term (in years) |
|
3.0 – 4.5 |
|
|
1.0 – 4.5 |
|
|
2.5 – 4.6 |
|
|||
|
Risk-free interest rate (%) |
|
|
1.5 |
% |
|
|
1.1 |
% |
|
|
1.2 |
% |
|
Expected volatility (%) |
|
|
49 |
% |
|
|
54 |
% |
|
|
51 |
% |
|
Dividend yield (%) |
|
|
0.0 |
% |
|
|
0.0 |
% |
|
|
0.0 |
% |
|
Weighted average fair value/share at grant date |
|
$ |
2.26 |
|
|
$ |
2.23 |
|
|
$ |
1.84 |
|
115
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The status of our stock options are summarized as follows:
|
|
|
Number Of Shares |
|
|
Weighted Average Exercise Price |
|
||
|
Outstanding at December 31, 2014 |
|
|
4,012,096 |
|
|
$ |
2.04 |
|
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
1,819,000 |
|
|
|
4.90 |
|
|
Exercised |
|
|
(492,091 |
) |
|
|
1.45 |
|
|
Canceled |
|
|
(12,500 |
) |
|
|
3.19 |
|
|
Outstanding at December 31, 2015 |
|
|
5,326,505 |
|
|
|
3.07 |
|
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
2,617,526 |
|
|
|
7.14 |
|
|
Exercised |
|
|
(2,483,519 |
) |
|
|
1.69 |
|
|
Canceled |
|
|
(324,402 |
) |
|
|
3.99 |
|
|
Outstanding at December 31, 2016 |
|
|
5,136,110 |
|
|
|
5.76 |
|
|
|
|
|
|
|
|
|
|
|
|
Granted |
|
|
2,119,498 |
|
|
|
7.60 |
|
|
Exercised |
|
|
(565,569 |
) |
|
|
3.84 |
|
|
Canceled |
|
|
(347,513 |
) |
|
|
6.12 |
|
|
Outstanding at December 31, 2017 |
|
|
6,342,526 |
|
|
|
6.51 |
|
|
Exercisable at December 31, 2017 |
|
|
2,103,342 |
|
|
|
5.50 |
|
The number and weighted average grant-date fair values of options non-vested at the beginning and end of 2017, as well as options granted, vested and forfeited during the year was as follows:
|
|
|
Number of Options |
|
|
Weighted Average Grant Date Fair Value |
|
||
|
Non-vested at December 31, 2016 |
|
|
4,008,478 |
|
|
$ |
2.09 |
|
|
Granted in 2017 |
|
|
2,119,498 |
|
|
|
2.26 |
|
|
Vested in 2017 |
|
|
(1,584,554 |
) |
|
|
2.20 |
|
|
Forfeited in 2017 |
|
|
(304,237 |
) |
|
|
2.38 |
|
|
Non-vested at December 31, 2017 |
|
|
4,239,185 |
|
|
|
2.29 |
|
The following table summarizes information about our options outstanding at December 31, 2017:
|
|
|
Options Outstanding |
|
|
Options Exercisable |
|
||||||||||||||||||
|
Range of Exercise Prices ($) |
|
Number Outstanding |
|
|
Weighted Average Remaining Contractual Life (Years) |
|
|
Weighted Average Exercise Price |
|
|
Number Exercisable |
|
|
Weighted Average Remaining Contractual Life (Years) |
|
|
Weighted Average Exercise Price |
|
||||||
|
1.47 – 4.00 |
|
|
296,001 |
|
|
|
0.97 |
|
|
$ |
3.53 |
|
|
|
273,501 |
|
|
|
0.95 |
|
|
$ |
3.52 |
|
|
4.01 – 5.00 |
|
|
1,600,334 |
|
|
|
2.23 |
|
|
|
4.76 |
|
|
|
1,044,500 |
|
|
|
2.20 |
|
|
|
4.75 |
|
|
5.01 – 7.00 |
|
|
513,832 |
|
|
|
2.97 |
|
|
|
6.59 |
|
|
|
158,257 |
|
|
|
2.87 |
|
|
|
6.49 |
|
|
7.01 – 7.50 |
|
|
1,901,528 |
|
|
|
3.49 |
|
|
|
7.17 |
|
|
|
507,511 |
|
|
|
3.30 |
|
|
|
7.15 |
|
|
7.51 – 8.00 |
|
|
1,644,999 |
|
|
|
4.26 |
|
|
|
7.55 |
|
|
|
37,500 |
|
|
|
2.98 |
|
|
|
7.86 |
|
|
8.01 – 9.47 |
|
|
385,832 |
|
|
|
3.93 |
|
|
|
8.47 |
|
|
|
82,073 |
|
|
|
3.65 |
|
|
|
8.39 |
|
|
|
|
|
6,342,526 |
|
|
|
3.24 |
|
|
|
6.52 |
|
|
|
2,103,342 |
|
|
|
2.43 |
|
|
|
5.50 |
|
As of December 31, 2017, the aggregate intrinsic value of all stock options outstanding and expected to vest was approximately $14.9 million and the aggregate intrinsic value of currently exercisable stock options was approximately $7.1 million. The intrinsic value of each option share is the difference between the fair market value
116
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
of NeoGenomic’s common stock and the exercise price of such option share to the extent it is “in-the-money”. Aggregate intrinsic value represents the value that would have been received by the holders of in-the-money options had they exercised their options on the last trading day of the year and sold the underlying shares at the closing stock price on such day. The intrinsic value calculation is based on the $8.86 closing stock price of NeoGenomics Common Stock on December 29, 2017, the last trading day of 2017. The total number of in-the-money options outstanding and exercisable as of December 31, 2017 was approximately 2.1 million.
The total intrinsic value of options exercised during the years ended December 31, 2017, 2016 and 2015 was approximately $2,772,000, $15,003,000 and $2,470,000, respectively. Intrinsic value of exercised shares is the total value of such shares on the date of exercise less the cash received from the option holder to exercise the options. The total cash proceeds received from the exercise of stock options was approximately $2,170,000, $4,179,000 and $714,000 for the years ended December 31, 2017, 2016 and 2015, respectively.
The total fair value of options granted during the years ended December 31, 2017, 2016 and 2015 was approximately $4,782,000, $6,493,000 and $3,347,000, respectively. The total fair value of option shares vested during the years ended December 31, 2017, 2016 and 2015 was approximately $3,617,000, $2,165,000 and $871,000.
We recognize stock-based compensation expense using the straight-line basis over the awards’ requisite service periods for employees and variably for non-employees due to the market-to-market adjustments at the end of each reporting period. Stock compensation cost recognized for the years ended December 31, 2017, 2016 and 2015 related to stock options was approximately $5,024,000, $4,978,000 and $2,889,000, respectively. As of December 31, 2017, there was approximately $5,056,000 of total unrecognized stock-based compensation cost related to unvested stock options granted under the Amended Plan. This cost is expected to be recognized over a weighted-average period of 1.0 years.
Employee Stock Purchase Plan
Effective January 1, 2007, the Company began sponsoring an Employee Stock Purchase Plan (“ESPP”), under which eligible employees could purchase common stock, by means of limited payroll deductions, at a 5% discount from the fair market value. In accordance with ASC Topic 718-50, Compensation – Stock Compensation – Employee Share Purchase Plans, the ESPP was considered non-compensatory and did not require the recognition of compensation cost because the discount offered to employees did not exceed 5%.
On May 25, 2017, the Company amended the ESPP, increasing the discount from 5% to 15%. As a result of this change, we have recorded stock-based compensation expense related to the ESPP for the period ended December 31, 2017 in the amount of approximately $98,000. Shares issued pursuant to this plan were 108,599, 98,672 and 73,958 for the years ended December 31, 2017, 2016 and 2015, respectively.
Common Stock Warrants
From time to time, the Company issues warrants to purchase its common stock. These warrants have been issued for consulting services, in connection with the Company’s credit facilities and sales of its common stock and in connection with employment agreements and for compensation to directors. These warrants are valued using trinomial lattice pricing model and using the volatility, market price, strike price, risk-free interest rate and dividend yield appropriate at the date the warrants were issued. There are no warrants outstanding as of December 31, 2017.
117
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
On January 9, 2012, we granted performance incentive warrants to Dr. Albitar to purchase 200,000 shares of the Company’s common stock (the “Albitar Warrants”) at an exercise price per share of $1.43. During the year ended December 31, 2016, all of these warrants were fully vested and exercised. The warrants were scheduled to expire on January 9, 2017. Warrant compensation expense (gain) for these warrants is recorded in research and development as the expense is related to performance based warrants to a non-employee. We recorded no warrant compensation expense for the year ended December 31, 2017, a gain of $10,000 for the year ended December 31, 2016 and approximately $422,000 for the year ended December 31, 2015.
On May 3, 2010, warrants to purchase 450,000 shares of common stock at an exercise price of $1.50 per share were granted to Mr. Steven C. Jones (see Note M). These warrants were subject to time and performance requirements, and were fully vested as of December 31, 2016. These warrants were sold to a third party in September of 2016 and subsequently exercised in March of 2017. There were no stock compensation expenses related to these warrants for the years ended December 31, 2017, 2016 or 2015.
Warrant activity is summarized as follows:
|
|
|
Shares |
|
|
Weighted Average Exercise Price |
|
||
|
Warrants outstanding, December 31, 2014 |
|
|
650,000 |
|
|
$ |
1.24 |
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
Exercised |
|
|
— |
|
|
|
— |
|
|
Expired |
|
|
— |
|
|
|
— |
|
|
Cancelled |
|
|
— |
|
|
|
— |
|
|
Warrants outstanding, December 31, 2015 |
|
|
650,000 |
|
|
|
1.48 |
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
Exercised |
|
|
(200,000 |
) |
|
|
— |
|
|
Expired |
|
|
— |
|
|
|
— |
|
|
Cancelled |
|
|
— |
|
|
|
— |
|
|
Warrants outstanding, December 31, 2016 |
|
|
450,000 |
|
|
|
1.50 |
|
|
Granted |
|
|
— |
|
|
|
— |
|
|
Exercised |
|
|
(450,000 |
) |
|
|
1.50 |
|
|
Expired |
|
|
— |
|
|
|
— |
|
|
Cancelled |
|
|
— |
|
|
|
— |
|
|
Warrants outstanding, December 31, 2017 |
|
|
- |
|
|
$ |
- |
|
|
Warrants exercisable at December 31, 2017 |
|
|
- |
|
|
$ |
- |
|
Note L – Commitments and Contingencies
Operating Leases
The Company leases its laboratory and office facilities under non-cancelable operating leases. These operating leases expire at various dates through December 2022 and generally require the payment of real estate taxes, insurance, maintenance, utility and operating costs. The Company has approximately 51,000 square feet of office and laboratory space at our corporate headquarters in Fort Myers, Florida. In addition, we maintain laboratory and office space in Aliso Viejo, and Fresno, California; Nashville, Tennessee; Houston, Texas; Tampa, Florida; Atlanta, Georgia and Rolle, Switzerland.
118
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
The following is a schedule of future minimum obligations under non-cancelable operating leases as of December 31, 2017 (in thousands):
|
Years ending December 31, |
|
|
|
|
|
2018 |
|
$ |
3,473 |
|
|
2019 |
|
|
2,890 |
|
|
2020 |
|
|
2,401 |
|
|
2021 |
|
|
762 |
|
|
2022 |
|
|
325 |
|
|
Thereafter |
|
|
- |
|
|
Total minimum lease payments |
|
$ |
9,851 |
|
Rent expense for the years ended December 31, 2017, 2016 and 2015 was approximately $4.7 million, $4.2 million and $1.9 million, respectively and is included in costs of revenues and in general and administrative expenses, depending on the allocation of work space in each facility. Certain of the Company’s facility leases include rent escalation clauses. The Company normalizes rent expense on a straight-line basis for known changes in lease payments over the life of the lease.
Purchase Commitments
The Company has agreements in place to purchase a specified level of reagents from certain vendors. These purchase commitments expire at various dates through 2020. The purchase commitments as of December 31, 2017 are as follows (in thousands):
|
Years ending December 31, |
|
|
|
|
|
2018 |
|
$ |
942 |
|
|
2019 |
|
|
838 |
|
|
2020 |
|
|
378 |
|
|
Total purchase commitments |
|
$ |
2,158 |
|
Capital Lease Obligations
The Company’s capital lease obligations expire at various times through 2020 and the weighted average interest rates under such leases approximated 5.20% at December 31, 2017. Some of our leases contain bargain purchase options that allow us to purchase the leased property for a minimal amount upon the expiration of the lease term. The remaining leases have purchase options at fair market value. See Note F for more information about future minimum lease payments under capital lease obligations, including those described above. Property and equipment acquired under capital lease agreements (see Note C) are pledged as collateral to secure the performance of the future minimum lease payments shown in Note F.
Employment Contracts
The agreements with our Chief Executive Officer, Chief Medical Officer, Clinical Services President, Vice President of Operations, Chief Information Officer and Chief Financial Officer contain some or all of the following:
|
|
• |
Clauses that allow for continuous automatic extensions of one year unless timely written notice terminating the contract is provided to such officers (as defined in the agreements). |
|
|
• |
Clauses that provide for accelerated vesting of the options granted pursuant to such agreements at the time of certain changes of control of the Company. |
|
|
• |
Clauses that provided for 6-12 months of severance benefits in the event that such officers are terminated without “cause” (as defined in the agreements) by the Company. The base salaries for these officers in 2017 approximate $2.5 million. |
119
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
Note M – Related Party Transactions
During the years ended December 31, 2017, 2016 and 2015, Steven C. Jones, a director of the Company, earned approximately $247,000, $263,000 and $261,500, respectively, for various consulting work performed in connection with his duties as an Executive Vice President and received reimbursement of incurred expenses. Mr. Jones also earned $31,912, $85,000 and $578,900 as payment of bonuses for the periods indicated above. The bonus earned for the year ended December 31, 2015 was comprised of $500,000 in recognition of the services provided in connection with the Company’s acquisition of Clarient, Inc. and the related financing. This amount was paid to Aspen Capital Advisors, LLC (“Aspen”) for which Mr. Jones is a managing director, pursuant to a consulting agreement entered into between Aspen and the Company on November 11, 2015. The remaining $78,900 was earned as part of a management incentive plan.
On May 25, 2017, the Company granted Mr. Jones 10,000 stock options to purchase shares of parent common stock. The options were granted at a price of $7.27 per share and had a weighted average fair market value of $2.47 per option. The options vest ratably over the next three years on each anniversary date. In addition, the Company granted Mr. Jones 8,667 shares of restricted common stock. Such restricted common stock vests ratably over each of the subsequent three quarters so long as he continues to serve as a member of the Board of Directors. The fair market value per share was deemed to be $63,009 or $7.27 per share, which was the closing price of the Parent’s common stock on the day before the grant was approved by the compensation committee of the Board of Directors.
On April 20, 2016, the Company granted Mr. Jones 100,000 stock options to purchase shares of parent common stock. The options were granted at a price of $7.15 per share and had a weighted average fair market value of $2.50 per option. The options vest ratably over the next three years on each anniversary date. These options were accounted for as granted to a non-employee as they relate to his services to the Company as a consultant.
On May 4, 2015, the Company granted Mr. Jones 225,000 stock options to purchase shares of parent common stock. The options were granted at a price of $4.78 per share and had a weighted average fair market value of $1.80 per option. The options vest ratably over the next three years on each anniversary date. 10,000 of the options were accounted for as granted to a Director of the Company, consistent with similar grants at that time to other Directors. The remaining 215,000 stock options have been accounted for as granted to a non-employee as they relate to his services to the Company as a consultant.
On May 3, 2010, the Company entered into a consulting agreement (the “Consulting Agreement”) with Mr. Jones whereby Mr. Jones would continue to provide consulting services to the Company in the capacity of Executive Vice President of Finance. On May 3, 2010, the Company also entered into a warrant agreement with Mr. Jones and it issued a warrant to purchase 450,000 shares of the Company’s common stock, which were all vested as of December 31, 2016 and fully exercised at December 31, 2017.
On November 4, 2016, the Company entered into an amended and restated consulting agreement (the “Amended and Restated Consulting Agreement”) with Mr. Jones. The Amended and Restated Consulting Agreement has an initial term of November 4, 2016 through April 30, 2020, which initial term automatically renews for additional one year periods unless either party provides notice of termination at least three months prior to the expiration of the initial term or any renewal term. In addition, the Company has the right to terminate the Amended and Restated Consulting Agreement by giving written notice to Mr. Jones the year prior to the effective date of termination. Mr. Jones has the right to terminate the Amended and Restated Consulting Agreement by giving written notice to the Company three months prior to the proposed termination date, provided, however, Mr. Jones is required to provide an additional three months of transition services to the Company upon reasonable request by the Company. The Amended and Restated Consulting Agreement specifies monthly base retainer compensation of $21,666 per month until April 30, 2017; $15,000 per month from May 1, 2017 until April 30, 2018; $12,500 per month from May 1, 2018 until April 30, 2019; and $10,000 per month thereafter. Mr. Jones is also eligible to receive a cash bonus based on the achievement of certain performance metrics with a target of 35% of his base retainer for any given fiscal year. Such bonus is eligible to be increased to up to 150% of the target bonus in any fiscal year in which he meets certain performance thresholds established by the CEO of the Company and approved by the Board of Directors.
.
120
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
We maintain a defined-contribution 401(k) retirement plan covering substantially all employees (as defined). Our employees may make voluntary contributions to the plan, subject to limitations based on IRS regulations and compensation. In addition, we match any employees’ contributions at the rate of 75% of every dollar contributed up to 4% of the respective employee’s salary (3% maximum Company match). Effective, January 1, 2017 this benefit increased to 100% of every dollar contributed up to 3% of the respective employee’s compensation and an additional 50% of every dollar contributed on the next 2% of compensation (4% maximum Company match). We made matching contributions of approximately $2,470,000, $1,660,000 and $493,000 during the years ended December 31, 2017, 2016 and 2015, respectively.
Note O – Equity Transactions
Restricted Stock Issued to Ascend Genomics
As discussed in Note E, the Company issued 450,000 shares of restricted common stock as consideration for the purchase of a customer list in August 2017. The restriction prohibits Ascend from registering and trading the shares for a period of six months from the issuance date.
Common Stock Issued to GE Medical
As discussed in Note D, on December 30, 2015, the Company issued 15,000,000 shares of common stock as consideration for the acquisition of Clarient. The common stock includes restrictions imposed on the holder in the Investor Board Rights, Lockup and Standstill Agreement.
Preferred Stock Issued to GE Medical
As discussed in Note D, on December 30, 2015 the Company issued 14,666,667 shares of Series A Preferred Stock as consideration for the acquisition of Clarient. In 2016, the Company redeemed 8,066,667 shares of the Series A Preferred Stock outstanding leaving a balance of 6,600,000 shares outstanding as of December 31, 2016. In 2017, the Company issued 264,000 additional shares of Preferred Stock as a PIK dividend resulting in a balance of 6,864,000 shares outstanding as of December 31, 2017.
Restricted Stock Awards
On May 25, 2017, the Company granted each of the six independent directors of the Board of Directors of the Parent (the “Board”); 8,667 shares of restricted common stock. Such restricted common stock vests ratably over each of the subsequent three quarters so long as the director continues to serve as a member of the Board. The fair market value of each grant of restricted common stock on the award date was deemed to be $63,009 or $7.27 per share, which was the closing price of Parent’s common stock on the day before the grant was approved by the compensation committee of the Board. In addition, the compensation committee of the Board approved 320,709 shares of restricted stock to be granted to other executives. The fair market value of share of restricted common stock on the award date was deemed to be $7.27.
On July 28, 2016, the Company granted each of the six independent directors of Parent 5,072 shares of restricted common stock. Such restricted common stock vests ratably over each of the subsequent three quarters so long as the director continues to serve as a member of the Board. The fair market value of each grant of restricted common stock on the award date was deemed to be $46,257 or $9.12 per share, which was the closing price of Parent’s common stock on the day before the grant was approved by the compensation committee of the Board.
On April 20, 2016, the Company granted each of six independent directors of Parent 2,150 shares of restricted common stock. Such restricted common stock vests ratably over each of the subsequent four quarters so long as the director continues to serve as a member of the Board. The fair market value of each grant of restricted common stock on the award date was deemed to be $15,050 or $7.00 per share, which was the closing price of Parent’s common stock on the day before the grant was approved by the compensation committee of the Board.
On June 16, 2015, the Company granted each of the two newly elected independent directors of Parent 1,560 shares of restricted common stock. Such restricted common stock vests ratably over each of the subsequent three quarters
121
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
so long as the director continues to serve as a member of the Board. The fair market value of each grant of restricted common stock on the award date was deemed to be $9,079 or $5.82 per share, which was the closing price of Parent’s common stock on the day before the grant was approved by the compensation committee of the Board.
On April 16, 2015, the Company granted each of the four independent directors of Parent each 2,080 shares of restricted common stock. Such restricted common stock vests ratably over each of the subsequent three quarters so long as the director continues to serve as a member of the Board. The fair market value of each grant of restricted common stock on the award date was deemed to be $10,025 or $4.82 per share, which was the closing price of Parent’s common stock on the day before the grant was approved by the compensation committee of the Board.
The number and weighted average grant date fair values of restricted non-vested common stock at the beginning and end of 2017, 2016 and 2015, as well as stock awards granted, vested and forfeited during the year are as follows:
|
|
|
Number of Restricted Shares |
|
|
Weighted Average Grant Date Fair Value |
|
||
|
Nonvested at December 31, 2014 |
|
|
128,375 |
|
|
$ |
3.06 |
|
|
Granted in 2015 |
|
|
11,440 |
|
|
|
5.08 |
|
|
Vested in 2015 |
|
|
(12,820 |
) |
|
|
4.56 |
|
|
Forfeited in 2015 |
|
|
— |
|
|
|
— |
|
|
Nonvested at December 31, 2015 |
|
|
126,995 |
|
|
|
3.10 |
|
|
Granted in 2016 |
|
|
43,332 |
|
|
|
8.49 |
|
|
Vested in 2016 |
|
|
(33,083 |
) |
|
|
8.13 |
|
|
Forfeited in 2016 |
|
|
— |
|
|
|
— |
|
|
Nonvested at December 31, 2016 |
|
|
137,244 |
|
|
|
3.59 |
|
|
Granted in 2017 |
|
|
372,711 |
|
|
|
7.27 |
|
|
Vested in 2017 |
|
|
(182,744 |
) |
|
|
4.50 |
|
|
Forfeited in 2017 |
|
|
— |
|
|
|
— |
|
|
Nonvested at December 31, 2017 |
|
|
327,211 |
|
|
|
7.27 |
|
Note P – Impairment
During the fourth quarter of 2016, as part of our annual impairment assessment, it was determined that the carrying amount of certain intangible assets exceeded fair value and were impaired.
The following table reconciles the asset impairment charges (in thousands), which are recognized in operating expenses in our consolidated statement of operations:
|
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Impairment of HDC Assets |
|
$ |
- |
|
|
$ |
1,902 |
|
|
$ |
- |
|
|
Impairment of Path Logic Assets |
|
|
- |
|
|
|
1,562 |
|
|
|
- |
|
|
Total Impairment |
|
$ |
- |
|
|
$ |
3,464 |
|
|
$ |
- |
|
HDC Assets
This impairment charge is related to the Master License Agreement with Health Discovery Corporation. This impairment charge writes off the HDC intangible assets associated with SVM, LDT, flow cytometry and cytogenetics technologies. The impairment is primarily the result of the lack of revenues to date, and the disputed license termination notification received from HDC. Based on this analysis, the Company determined that the assets were fully impaired, and an impairment loss was recorded for the unamortized balance of these assets in the amount of $1.9 million.
122
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
This impairment charge is associated with our Path Logic intangible assets, consisting of customer relationships. Based on the analysis performed, this asset is fully impaired.
Note Q – Segment Information
We have two primary types of customers, clinical and pharma. Our clinical customers include community based pathology practices, oncology groups, hospitals and academic centers. Our pharma customers include pharmaceutical companies to whom we provide testing and other services to support their studies and clinical trials. We have historically presented these customer types as one operating segment.
In the fourth quarter of 2017, changes were made in the information provided to our Chief Operating Decision Maker (“CODM”); greater detail was provided regarding the performance of our Pharma business and our Clinical business as there was an increased focus on this financial data due to the growth of our Pharma business. Our CODM also changed the way he was using this financial information to make strategic decisions regarding allocation of resources and evaluating performance of the Company. This resulted in a change in our operating segments to align with how the CODM views our business which resulted in two operating segments; a Pharma Services segment and a Clinical Services segment.
We have presented the financial information reviewed by the CODM including revenues, cost of revenue and gross margin for each of our operating segments. The segment information presented in these financial statements has been conformed to present segments on this revised basis for all prior periods. Assets are not presented at the segment level as that information is not used by the CODM.
The following table summarizes segment information for the years ended December 31, 2017, 2016 and 2015 (in thousands).
|
|
|
For the Years Ended December 31, |
|
|||||||||
|
|
|
2017 |
|
|
2016 |
|
|
2015 |
|
|||
|
Net revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical testing |
|
$ |
231,748 |
|
|
$ |
222,015 |
|
|
$ |
98,595 |
|
|
Pharma Services |
|
|
26,863 |
|
|
|
22,068 |
|
|
|
1,207 |
|
|
Total Revenue |
|
$ |
258,611 |
|
|
$ |
244,083 |
|
|
$ |
99,802 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical testing |
|
$ |
121,785 |
|
|
$ |
120,437 |
|
|
$ |
55,802 |
|
|
Pharma Services |
|
|
16,510 |
|
|
|
13,267 |
|
|
|
244 |
|
|
Total Cost of Revenue |
|
$ |
138,295 |
|
|
$ |
133,704 |
|
|
$ |
56,046 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross margin: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Clinical testing |
|
$ |
109,963 |
|
|
$ |
101,578 |
|
|
$ |
42,793 |
|
|
Pharma Services |
|
|
10,353 |
|
|
|
8,801 |
|
|
|
963 |
|
|
Total Gross Margin |
|
$ |
120,316 |
|
|
$ |
110,379 |
|
|
$ |
43,756 |
|
Note R – Subsequent Events
On February 26, 2018, the Compensation Committee of the Board of Directors granted 1,585,000 options to certain executive officers and key employees of the Company. The options were granted at a price of $8.03 per share and had a weighted average fair market value of $2.60 per option for a total fair market value of approximately $4.1 million. We expect our stock option compensation expense to increase by approximately $2.1 million, $1.4 million, $566,000, and $71,000 in the years ended December 31, 2018, 2019, 2020 and 2021, respectively.
123
NEOGENOMICS, INC.
NOTES TO THE FINANCIAL STATEMENTS
December 31, 2017, 2016 and 2015
Note S – Quarterly Financial Data (Unaudited)
Supplementary Data
Selected Quarterly Financial Data (unaudited) (in thousands, except per share data)
|
|
|
For the Quarters Ended |
|
|
Total |
|
||||||||||||||
|
|
|
03/31/17 |
|
|
06/30/17 |
|
|
09/30/17 |
|
|
12/31/17 |
|
|
2017 |
|
|||||
|
Net revenues |
|
$ |
61,676 |
|
|
$ |
66,090 |
|
|
$ |
63,052 |
|
|
$ |
67,792 |
|
|
$ |
258,611 |
|
|
Gross profit |
|
$ |
27,196 |
|
|
$ |
31,178 |
|
|
$ |
28,810 |
|
|
$ |
33,132 |
|
|
$ |
120,316 |
|
|
Net income (loss) |
|
$ |
(654 |
) |
|
$ |
(43 |
) |
|
$ |
(5,100 |
) |
|
$ |
4,951 |
|
|
$ |
(846 |
) |
|
Deemed dividends on preferred stock and amortization of preferred stock beneficial conversion feature |
|
$ |
2,566 |
|
|
$ |
2,639 |
|
|
$ |
2,651 |
|
|
$ |
2,691 |
|
|
$ |
10,547 |
|
|
Net income (loss) available to common stockholders |
|
$ |
(3,220 |
) |
|
$ |
(2,682 |
) |
|
$ |
(7,751 |
) |
|
$ |
2,260 |
|
|
$ |
(11,393 |
) |
|
Net income (loss) per common share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.04 |
) |
|
$ |
(0.03 |
) |
|
$ |
(0.10 |
) |
|
$ |
0.03 |
|
|
$ |
(0.14 |
) |
|
Diluted |
|
$ |
(0.04 |
) |
|
$ |
(0.03 |
) |
|
$ |
(0.10 |
) |
|
$ |
0.03 |
|
|
$ |
(0.14 |
) |
|
Weighted average common shares outstanding – Basic |
|
|
78,650 |
|
|
|
79,413 |
|
|
|
79,617 |
|
|
|
86,676 |
|
|
|
79,426 |
|
|
Weighted average shares outstanding – Diluted |
|
|
78,650 |
|
|
|
79,413 |
|
|
|
79,617 |
|
|
|
88,611 |
|
|
|
79,426 |
|
|
|
|
For the Quarters Ended |
|
|
Total |
|
||||||||||||||
|
|
|
03/31/16 |
|
|
06/30/16 |
|
|
09/30/16 |
|
|
12/31/16 |
|
|
2016 |
|
|||||
|
Net revenues |
|
$ |
59,704 |
|
|
$ |
63,129 |
|
|
$ |
60,761 |
|
|
$ |
60,489 |
|
|
$ |
244,083 |
|
|
Gross profit |
|
$ |
27,173 |
|
|
$ |
28,605 |
|
|
$ |
27,345 |
|
|
$ |
27,256 |
|
|
$ |
110,379 |
|
|
Net income (loss) |
|
$ |
155 |
|
|
$ |
413 |
|
|
$ |
(67 |
) |
|
$ |
(6,224 |
) |
|
$ |
(5,723 |
) |
|
Deemed dividends on preferred stock and amortization of preferred stock beneficial conversion feature |
|
$ |
5,567 |
|
|
$ |
5,567 |
|
|
$ |
5,567 |
|
|
$ |
7,973 |
|
|
$ |
24,674 |
|
|
Net (loss) available to common stockholders |
|
$ |
(5,412 |
) |
|
$ |
(5,154 |
) |
|
$ |
(5,634 |
) |
|
$ |
(14,197 |
) |
|
$ |
(30,397 |
) |
|
Net (loss) per common share: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
$ |
(0.07 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.18 |
) |
|
$ |
(0.39 |
) |
|
Diluted |
|
$ |
(0.07 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.18 |
) |
|
$ |
(0.39 |
) |
|
Weighted average common shares outstanding – Basic |
|
|
76,068 |
|
|
|
77,448 |
|
|
|
78,145 |
|
|
|
78,490 |
|
|
|
77,542 |
|
|
Weighted average shares outstanding – Diluted |
|
|
76,068 |
|
|
|
77,448 |
|
|
|
78,145 |
|
|
|
78,490 |
|
|
|
77,542 |
|
End of Financial Statements
124
NEOGENOMICS, INC.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of our disclosure controls and procedures as of December 31, 2017. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of December 31, 2017, our disclosure controls and procedures were (1) effective in that they were designed to ensure that material information relating to us, and information required to be disclosed in our reports to the SEC, including our consolidated subsidiaries, is made known to our Chief Executive Officer and Chief Financial Officer by others within those entities, particularly during the period in which this report was being prepared, as appropriate to allow timely discussions and decisions regarding required disclosure therein and (2) effective, in that they provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms.
Management’s Report on Internal Control over Financial Reporting
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) or 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, our principal executive and principal financial officer and effected by the Company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures: (1) that pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, however, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Our management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2017. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework (2013 Framework). Based on our assessment, management, with the participation of our Chief Executive Officer and Chief Financial Officer, concluded that, as of December 31, 2017, our internal control over financial reporting was effective based on those criteria at the reasonable assurance level. The effectiveness of our internal control over financial reporting as of December 31, 2017 has been audited by Crowe Horwath LLP, an independent registered public accounting firm, as stated and attested to in their report that is included in Item 8.
Changes in Internal Control over Financial Reporting
During the quarter ended December 31, 2017, we implemented changes in the Company’s internal control over financial reporting. These changes include re-design of review and approval controls over the accurate recording, presentation, and disclosure of revenue. In addition, we have implemented new controls surrounding our monthly revenue closing process. These changes have not materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
None.
125
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item 10 will be included under the captions “Election of Directors”, “Information as to Nominees and Other Directors”, “Information Regarding Meetings and Committees of the Board”, “Section 16(a) Beneficial Ownership Reporting Compliance” and as otherwise, set forth in the Company’s 2018 Proxy Statement and is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 will be included under the captions “Executive Compensation and Other Information” and “Compensation Committee Interlocks and Insider Participation” and as otherwise set forth in the Company’s 2018 Proxy Statement and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item 12 will be included under the captions “Security Ownership” and “Equity Compensation Plan Information” and as otherwise set forth in the Company’s 2018 Proxy Statement and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
The information required by this Item 13 will be included under the captions “Certain Relationships and Related Party Transactions” and “Information Regarding Meetings and Committees of the Board” and as otherwise set forth in the Company’s 2018 Proxy Statement and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this Item 14 will be included under the caption “Independent Auditors” and as otherwise set forth in the Company’s 2018 Proxy Statement and is incorporated herein by reference.
126
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
Financial Statements: See Index to Consolidated Financial Statements under Part II, Item 8 of this Annual Report on Form 10-K
|
Exhibit |
|
Description of Exhibit |
|
Location |
|
|
|
|
||
|
2.1 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on October 26, 2015 |
|
|
|
|
|
|
|
|
2.2 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on December 31, 2015 |
|
|
|
|
|
|
|
|
3.1 |
|
|
Incorporated by reference to the Company’s Registration Statement on Form SB-2 as filed with the SEC on February 10, 1999 |
|
|
|
|
|
||
|
3.2 |
|
Amendment to Articles of Incorporation filed with the Nevada Secretary of State on January 3, 2002 |
|
Incorporated by reference to the Company’s Annual Report on Form 10-KSB for the year ended December 31, 2002, as filed with the SEC on May 20, 2003 |
|
|
|
|
||
|
3.3 |
|
Amendment to Articles of Incorporation filed with the Nevada Secretary of State on April 11, 2003 |
|
Incorporated by reference to the Company’s Annual Report on Form 10-KSB for the year ended December 31, 2002, as filed with the SEC on May 20, 2003 |
|
|
|
|
|
|
|
3.4 |
|
Amendment to Articles of Incorporation filed with the Nevada Secretary of State on December 28, 2015 |
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on December 31, 2015 |
|
|
|
|
||
|
3.5 |
|
Certificate of Designation of Series A Convertible Preferred Stock |
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on December 31, 2015 |
|
|
|
|
|
|
|
3.6 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K, as filed with the SEC on October 17, 2014 |
|
|
|
|
|
|
|
|
3.7 |
|
|
Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2015, as filed with the SEC on November 6, 2015 |
|
|
|
|
|
|
|
|
10.1 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on March 30, 2005 |
|
|
|
|
|
||
|
10.2 |
|
|
Incorporated by reference to the Company’s Annual Report on Form 10-KSB for the year ended December 31, 2005, as filed with the SEC on April 3, 2006 |
|
|
|
|
|
||
|
10.3 |
|
|
Incorporated by reference to the Company’s Annual Report on Form 10-KSB for the year ended December 31, 2005, as filed with the SEC on April 3, 2006 |
|
|
|
|
|
||
|
10.4 |
|
|
Incorporated by reference to the Company’s Annual Report on Form 10-KSB for the year ended December 31, 2005, as filed with the SEC on April 3, 2006 |
|
|
|
|
|
|
|
|
10.5 |
|
|
Incorporated by reference to the Company’s Registration Statement on Form SB-2 as filed with the SEC on July 6, 2007 |
|
|
|
|
|
||
|
10.6 |
|
|
Incorporated by reference to the Company’s Registration Statement on Form SB-2 as filed with the SEC on July 6, 2007 |
|
|
|
|
|
||
127
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
Exhibit |
|
Description of Exhibit |
|
Location |
|
|
|
|
||
|
|
Employment Agreement, dated March 16, 2009 between Mr. Douglas M. VanOort and NeoGenomics, Inc. |
|
Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2010, as filed with the SEC on August 16, 2010 |
|
|
|
|
|
||
|
10.8 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on March 20, 2009 |
|
|
|
|
|
||
|
10.9* |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on November 3, 2009 |
|
|
|
|
|
||
|
10.10* |
|
Employment Letter dated November 3, 2009 between NeoGenomics Laboratories, Inc. and George Cardoza |
|
Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2010, as filed with the SEC on August 16, 2010 |
|
|
|
|
||
|
10.11 |
|
|
Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2016, as filed with the SEC on November 7, 2016 |
|
|
|
|
|
||
|
10.12 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on January 11, 2012 |
|
|
|
|
|
||
|
10.13* |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on January 11, 2012 |
|
|
|
|
|
|
|
|
10.14 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on January 11, 2012 |
|
|
|
|
|
||
|
10.15 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on January 11, 2012 |
|
|
|
|
|
||
|
10.16 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on January 11, 2012 |
|
|
|
|
|
||
|
10.17* |
|
Offer Letter between NeoGenomics Laboratories, Inc. and Steven Ross dated April 19, 2013 |
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on April 23, 2013 |
|
|
|
|
||
|
10.18 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on April 23, 2013 |
|
|
|
|
|
||
|
10.19 |
|
|
Incorporated by reference to Exhibit 2.1 to our Current Report on Form 8-K filed on July 11, 2014 |
|
|
|
|
|
||
|
10.20* |
|
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K as filed with the SEC on October 3, 2014 |
|
|
|
|
|
||
|
10.21 |
|
|
Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K as filed with the SEC on October 3, 2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
128
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
Exhibit |
|
Description of Exhibit |
|
Location |
|
|
|
|
||
|
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on December 31, 2015 |
||
|
|
|
|
||
|
10.23
|
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on December 31, 2015 |
|
|
|
|
|
|
|
|
10.24 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on December 31, 2015 |
|
|
|
|
|
|
|
|
10.25 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on December 31, 2015 |
|
|
|
|
|
|
|
|
10.26 |
|
Engagement Letter between Aspen Capital Advisors, LLC and NeoGenomics, Inc. dated November 11, 2015. |
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on November 17, 2015 |
|
|
|
|
|
|
|
10.27* |
|
Amended and Restated Equity Incentive Plan effective as of October 15, 2015. |
|
Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, as filed with the SEC on March 15, 2016 |
|
|
|
|
|
|
|
10.28* |
|
Amendment No. 1 of the Amended and Restated Equity Incentive Plan, effective as of May 25, 2017. |
|
Incorporated by reference to the Company’s Proxy Statement, dated April 24, 2017, as filed with the SEC on April 25, 2017 |
|
|
|
|
|
|
|
10.29
|
|
|
|
Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2016, as filed with the SEC on November 7, 2016 |
|
|
|
|
|
|
|
10.30 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on December 27, 2016 |
|
|
|
|
|
|
|
|
14.1 |
|
NeoGenomics, Inc. Code of Ethics for Senior Financial Officers and the Principal Executive Officer |
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on July 20, 2011 |
|
|
|
|
||
|
21.1 |
|
|
Provided herewith |
|
|
|
|
|
||
|
23.1 |
|
|
Provided herewith |
|
|
|
|
|
||
|
31.1 |
|
|
Provided herewith |
|
|
|
|
|
||
|
31.2 |
|
|
Provided herewith |
|
|
|
|
|
||
|
32.1** |
|
|
Provided herewith |
|
|
|
|
|
||
|
99.1 |
|
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on October 17, 2014 |
|
129
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
|
Exhibit |
|
Description of Exhibit |
|
Location |
|
|
|
|
||
|
|
|
|
||
|
|
|
|
||
|
99.2 |
|
Charter of the Nominating and Corporate Governance Committee |
|
Incorporated by reference to the Company’s Current Report on Form 8-K as filed with the SEC on October 17, 2014 |
|
|
|
|
|
|
|
101 |
|
The following materials from the Company’s Annual Report on Form 10-K for the year ended December 31, 2017 formatted in Extensible Business Reporting Language (XBRL): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Stockholders Equity (iv) the Consolidated Statements of Cash Flows and (v) related notes. |
|
Provided herewith |
|
|
† |
Portions of the exhibit have been omitted pursuant to a request for confidential treatment pursuant to Rule 24b-2 promulgated under the Exchange Act. The omitted information has been filed separately with the SEC. |
|
* |
Denotes a management contract or compensatory plan or arrangement. |
|
** |
The certification attached as Exhibit 32.1 that accompanies this Form 10-K is not deemed filed with the SEC and is not to be incorporated by reference into any filing of NeoGenomics, Inc. under the Securities Act or the Exchange Act, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing. |
130
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
Date: March 13, 2018 |
|
NEOGENOMICS, INC. |
||
|
|
|
|
|
|
|
|
|
By: |
|
/s/ Douglas M. VanOort |
|
|
|
Name: |
|
Douglas M. VanOort |
|
|
|
Title: |
|
Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signatures |
|
Title(s) |
|
Date |
|
|
|
|
|
|
|
/s/ Douglas M. VanOort |
|
Chairman of the Board and Chief Executive Officer (Principal Executive Officer) |
|
March 13, 2018 |
|
Douglas M. VanOort |
|
|
||
|
|
|
|
|
|
|
/s/ Steven C. Jones |
|
Executive Vice President and Director |
|
March 13, 2018 |
|
Steven C. Jones |
|
|
||
|
|
|
|
|
|
|
/s/ George A. Cardoza |
|
Chief Financial Officer (Principal Financial Officer) |
|
March 13, 2018 |
|
George A. Cardoza |
|
|
||
|
|
|
|
|
|
|
/s/ Kathryn B. McKenzie |
|
Principal Accounting Officer |
|
March 13, 2018 |
|
Kathryn B. McKenzie |
|
|
||
|
|
|
|
|
|
|
/s/ Lynn A. Tetrault |
|
Director |
|
March 13, 2018 |
|
Lynn A. Tetrault |
|
|
||
|
|
|
|
|
|
|
/s/ Raymond R. Hipp |
|
Director |
|
March 13, 2018 |
|
Raymond R. Hipp |
|
|
||
|
|
|
|
|
|
|
/s/ Bruce K. Crowther |
|
Director |
|
March 13, 2018 |
|
Bruce K. Crowther |
|
|
131